Bioprinting extracellular vesicles as a "cell-free" regenerative medicine approach
- PMID: 39697984
- PMCID: PMC11648406
- DOI: 10.20517/evcna.2023.19
Bioprinting extracellular vesicles as a "cell-free" regenerative medicine approach
Abstract
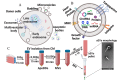
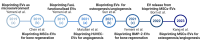
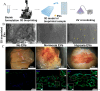
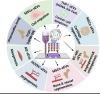
Regenerative medicine involves the restoration of tissue or organ function via the regeneration of these structures. As promising regenerative medicine approaches, either extracellular vesicles (EVs) or bioprinting are emerging stars to regenerate various tissues and organs (i.e., bone and cardiac tissues). Emerging as highly attractive cell-free, off-the-shelf nanotherapeutic agents for tissue regeneration, EVs are bilayered lipid membrane particles that are secreted by all living cells and play a critical role as cell-to-cell communicators through an exchange of EV cargos of protein, genetic materials, and other biological components. 3D bioprinting, combining 3D printing and biology, is a state-of-the-art additive manufacturing technology that uses computer-aided processes to enable simultaneous patterning of 3D cells and tissue constructs in bioinks. Although developing an effective system for targeted EVs delivery remains challenging, 3D bioprinting may offer a promising means to improve EVs delivery efficiency with controlled loading and release. The potential application of 3D bioprinted EVs to regenerate tissues has attracted attention over the past few years. As such, it is timely to explore the potential and associated challenges of utilizing 3D bioprinted EVs as a novel "cell-free" alternative regenerative medicine approach. In this review, we describe the biogenesis and composition of EVs, and the challenge of isolating and characterizing small EVs - sEVs (< 200 nm). Common 3D bioprinting techniques are outlined and the issue of bioink printability is explored. After applying the following search strategy in PubMed: "bioprinted exosomes" or "3D bioprinted extracellular vesicles", eight studies utilizing bioprinted EVs were found that have been included in this scoping review. Current studies utilizing bioprinted sEVs for various in vitro and in vivo tissue regeneration applications, including angiogenesis, osteogenesis, immunomodulation, chondrogenesis and myogenesis, are discussed. Finally, we explore the current challenges and provide an outlook on possible refinements for bioprinted sEVs applications.
Keywords: 3D bioprinting; bioprinted sEVs; regenerative medicine; small extracellular vesicles.
© The Author(s) 2023.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures



References
-
- Théry C, Witwer KW, Aikawa E, et al. Minimal information for studies of extracellular vesicles 2018 (MISEV2018): a position statement of the International Society for Extracellular Vesicles and update of the MISEV2014 guidelines. J Extracell Vesicles. 2018;7:1535750. doi: 10.1080/20013078.2018.1535750. - DOI - PMC - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
