Monosaccharide coating modulate the intracellular trafficking of gold nanoparticles in dendritic cells
- PMID: 39698001
- PMCID: PMC11652954
- DOI: 10.1016/j.mtbio.2024.101371
Monosaccharide coating modulate the intracellular trafficking of gold nanoparticles in dendritic cells
Abstract
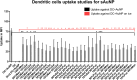
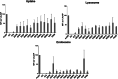
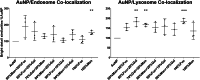
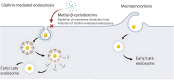
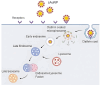
Dendritic cells (DCs) have emerged as a promising target for drug delivery and immune modulation due to their pivotal role in initiating the adaptive immune response. Gold nanoparticles (AuNPs) have garnered interest as a platform for targeted drug delivery due to their biocompatibility, low toxicity and precise control over size, morphology and surface functionalization. Our investigation aimed to elucidate the intracellular uptake and trafficking of AuNPs coated with different combinations of monosaccharides (mannose, galactose, and fucose) in DCs. We used 30 unique polymer-tethered monosaccharide combinations to coat 16 nm diameter spherical gold nanoparticles and investigated their effect on DCs phenotype, uptake, and intracellular trafficking. DCs internalized AuNPs coated with 100 % fucose, 100 % mannose, 90 % mannose +10 % galactose, and 80 % mannose +20 % galactose with highest efficiency. Flow cytometry analysis indicated that 100 % fucose-coated AuNPs showed increased lysosomal and endosomal contents compared to other conditions and uncoated AuNPs. Imaging flow cytometry further demonstrated that 100 % fucose-coated AuNPs had enhanced co-localization with lysosomes, while 100 % mannose-coated AuNPs exhibited higher co-localization with endosomes. Furthermore, our data showed that the uptake of carbohydrate-coated AuNPs predominantly occurred through receptor-mediated endocytosis, as evidenced by a marked reduction of uptake upon treatment of DCs with methyl-β-cyclodextrins, known to disrupt receptor-mediated endocytosis. These findings highlight the utility of carbohydrate coatings to enable more targeted delivery of nanoparticles and their payload to distinct intracellular compartments in immune cells with potential applications in drug delivery and immunotherapy.
Keywords: Carbohydrates; Dendritic cells; Gold nanoparticles; Immune modulation; Monosaccharides; Surface coating; T cells.
© 2024 The Authors.
Conflict of interest statement
The authors declare the following financial interests/personal relationships which may be considered as potential competing interests:Amir Ghaemmaghami and Morgan Alexander report financial support, administrative support, and travel were provided by the United Kingdom 10.13039/501100000266Engineering and Physical Sciences Research Council. Matthew I. Gibson reports financial support was provided by 10.13039/501100000781European Research Council. Nothing to declare If there are other authors, they declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures




Similar articles
-
Developing immune-regulatory materials using immobilized monosaccharides with immune-instructive properties.Mater Today Bio. 2020 Sep 30;8:100080. doi: 10.1016/j.mtbio.2020.100080. eCollection 2020 Sep. Mater Today Bio. 2020. PMID: 33205040 Free PMC article.
-
Endocytosis-driven gold nanoparticle fractal rearrangement in cells and its influence on photothermal conversion.Nanoscale. 2020 Nov 5;12(42):21832-21849. doi: 10.1039/d0nr05886f. Nanoscale. 2020. PMID: 33104150
-
The Shape of Nanostructures Encodes Immunomodulation of Carbohydrate Antigen and Vaccine Development.ACS Chem Biol. 2022 May 20;17(5):1122-1130. doi: 10.1021/acschembio.1c00998. Epub 2022 Apr 15. ACS Chem Biol. 2022. PMID: 35426652
-
Polymer decorated gold nanoparticles in nanomedicine conjugates.Adv Colloid Interface Sci. 2017 Nov;249:386-399. doi: 10.1016/j.cis.2017.01.007. Epub 2017 Feb 15. Adv Colloid Interface Sci. 2017. PMID: 28259207 Review.
-
Advances in Gold Nanoparticles: Synthesis, Functionalization Strategies, and Theranostic Applications in Cancer.Crit Rev Ther Drug Carrier Syst. 2024;41(6):1-56. doi: 10.1615/CritRevTherDrugCarrierSyst.2024046712. Crit Rev Ther Drug Carrier Syst. 2024. PMID: 38804553 Review.
References
-
- Hubbell J.A., Thomas S.N., Swartz M.A. Materials engineering for immunomodulation. Nature. 2009;462(7272):449–460. - PubMed
-
- Sampaolesi S., Nicotra F., Russo L. Glycans in nanomedicine, impact and perspectives. Future Med. Chem. 2019;11(1):43–60. - PubMed
-
- Ahmad S., Zamry A.A., Tan H.-T.T., Wong K.K., Lim J., Mohamud R. Targeting dendritic cells through gold nanoparticles: a review on the cellular uptake and subsequent immunological properties. Mol. Immunol. 2017;91:123–133. - PubMed
-
- Niikura K., Matsunaga T., Suzuki T., Kobayashi S., Yamaguchi H., Orba Y., Kawaguchi A., Hasegawa H., Kajino K., Ninomiya T., et al. Gold nanoparticles as a vaccine platform: influence of size and shape on immunological responses in vitro and in vivo. ACS Nano. 2013;7(5):3926–3938. - PubMed
LinkOut - more resources
Full Text Sources

