Advances in extracellular vesicle isolation methods: a path towards cell-type specific EV isolation
- PMID: 39698024
- PMCID: PMC11648483
- DOI: 10.20517/evcna.2023.14
Advances in extracellular vesicle isolation methods: a path towards cell-type specific EV isolation
Abstract
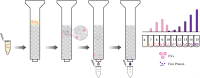
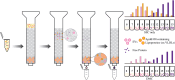
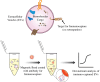
Extracellular vesicles are small, heterogenous, phospholipid-rich vesicles that are secreted by all cells into the extracellular space. They play a key role in intercellular communication because they can transport a variety of biomolecules such as proteins, lipids, and nucleic acids between cells. As categorized by the International Society of Extracellular Vesicles (ISEV), the term EV encompasses different sub-types, including exosomes, microvesicles, and apoptotic bodies, which differ in their size, origin, and cargo. EVs can be isolated from biological fluids such as blood, urine, and cerebrospinal fluid, and their biomolecular content can be analyzed to monitor the progression of certain diseases. Therefore, EVs can be used as a new source of liquid biomarkers for advancing novel diagnostic and therapeutic tools. Isolating and analyzing EVs can be challenging due to their nanoscopic size and low abundance. Several techniques have been developed for the isolation and characterization of EVs, including ultracentrifugation, density gradient separation, size-exclusion chromatography, microfluidics, and magnetic bead-based/affinity methods. This review highlights advances in EV isolation techniques in the last decade and provides a perspective on their advantages, limitations, and potential application to cell-type specific EV isolation in the future.
Keywords: Extracellular vesicles; biomarkers; cell-type specific; diagnostics; isolation methods.
© The Author(s) 2023.
Conflict of interest statement
Shami-shah A, Travis BG declare no known conflicts of interest. Walt DR has a financial interest in Quanterix, a company developing an ultrasensitive digital immunoassay platform; he is an inventor of the Simoa technology, a founder of the company, and a member of its board of directors. Walt DR ’s interests are reviewed and managed by BWH and Partners HealthCare in accordance with their conflict-of-interest policies.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
Research Materials
