Pathogenic single nucleotide polymorphisms in RhoA gene: Insights into structural and functional impacts on RhoA-PLD1 interaction through molecular dynamics simulation
- PMID: 39698059
- PMCID: PMC11653153
- DOI: 10.1016/j.crstbi.2024.100159
Pathogenic single nucleotide polymorphisms in RhoA gene: Insights into structural and functional impacts on RhoA-PLD1 interaction through molecular dynamics simulation
Abstract
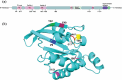
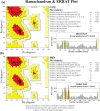
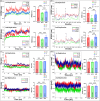
Molecular switches serve as key regulators of biological systems by acting as one of the crucial driving forces in the initiation of signal transduction pathway cascades. The Ras homolog gene family member A (RhoA) is one of the molecular switches that binds with GTP in order to cycle between an active GTP-bound state and an inactive GDP-bound state. Any aberrance in control over this circuit, particularly due to any perturbation in switching, leads to the development of different pathogenicity. Consequently, the single nucleotide polymorphisms (SNPs) within the RhoA gene, especially deleterious genetic variations, are crucial to study to forecast structural alteration and their functional impacts in light of disease onset. In this comprehensive study, we employed a range of computational tools to screen the deleterious SNPs of RhoA from 207 nonsynonymous SNPs (nsSNPs). By utilizing 7 distinct tools for further analysis, 8 common deleterious SNPs were sorted, among them 5 nsSNPs (V9G, G17E, E40K, A61T, F171L) were found to be in the highly conserved regions, with E40K and A61T at G2 and G3 motif of the GTP-binding domain respectively, indicating potential perturbation in GTP/GDP binding ability of the protein. RhoA-GDP complex interacts with the enzyme phospholipase, specifically PLD1, to regulate different cellular activities. PLD1 is also a crucial regulator of thrombosis and cancer. In that line of focus, our initial structural analysis of Y66H, A61T, G17E, I86N, and I151T mutations of RhoA revealed remarkable decreased hydrophobicity from which we further filtered out G17E and I86N which may have potential impact on the RhoA-GDP-PLD1 complex. Intriguingly, the comparative 250 ns (ns) molecular dynamics (MD) simulation of these two mutated complexes revealed overall structural instability and altered interaction patterns. Therefore, further investigation into these deleterious mutations with in vitro and in vivo studies could lead to the identification of potential biomarkers in terms of different pathogenesis and could also be utilized in personalized therapeutic targets in the long run.
Keywords: Deleterious SNP; Molecular dynamic simulation; Molecular switching; RhoA; RhoA-GDP-PLD1.
© 2024 The Authors. Published by Elsevier B.V.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures








References
-
- Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., Lindah E. GROMACS: high performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 2015;1–2:19–25. doi: 10.1016/J.SOFTX.2015.06.001. - DOI
-
- Abramson J., Adler J., Dunger J., Evans R., Green T., Pritzel A., Ronneberger O., Willmore L., Ballard A.J., Bambrick J., Bodenstein S.W., Evans D.A., Hung C.C., O'Neill M., Reiman D., Tunyasuvunakool K., Wu Z., Žemgulytė A., Arvaniti E., Beattie C., Bertolli O., Bridgland A., Cherepanov A., Congreve M., Cowen-Rivers A.I., Cowie A., Figurnov M., Fuchs F.B., Gladman H., Jain R., Khan Y.A., Low C.M.R., Perlin K., Potapenko A., Savy P., Singh S., Stecula A., Thillaisundaram A., Tong C., Yakneen S., Zhong E.D., Zielinski M., Žídek A., Bapst V., Kohli P., Jaderberg M., Hassabis D., Jumper J.M. Accurate structure prediction of biomolecular interactions with AlphaFold 3. Nature. 2024;630:493–500. doi: 10.1038/s41586-024-07487-w. 2024 630:8016. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

