Comprehensive method for producing high-affinity mouse monoclonal antibodies of various isotypes against (4-hydroxy-3-nitrophenyl)acetyl (NP) hapten
- PMID: 39698082
- PMCID: PMC11652855
- DOI: 10.1016/j.heliyon.2024.e40837
Comprehensive method for producing high-affinity mouse monoclonal antibodies of various isotypes against (4-hydroxy-3-nitrophenyl)acetyl (NP) hapten
Abstract
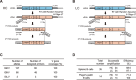
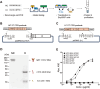
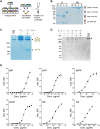
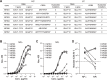
Monoclonal antibody (mAb) technology has significantly contributed to basic research and clinical settings for various purposes, including protective and therapeutic drugs. However, a rapid and convenient method to generate high-affinity antigen-specific mAbs has not yet been reported. Here, we developed a rapid, easy, and low-cost protocol for antigen-specific mAb production from single memory B cells. Using this method, high-affinity IgG1 mAbs specific to the hapten 4-hydroxy-3-nitrophenylacetyl (NP) were established from NP-CGG immunized C57BL/6 mice within 6 days. Our mAb production system allows flexible switching of IgG1 to any other isotype with the same paratope, enabling the absolute quantification of antigen-specific serum antibody titers and affinity maturation. Additionally, we established a protocol for the production of IgM and IgA, retaining their functional pentamer and dimer structures. This method is also effective against human antigens and pathogens, making it a powerful tool for mAb development in both research and clinical settings.
Keywords: 4-Hydroxy-3-nitrophenylacetyl (NP); Affinity maturation; B cell receptor; IgA; IgE; IgG; IgM; J chain; Memory B cells; Monoclonal antibody; Secretory component; Single cell.
© 2024 The Authors.
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


Similar articles
-
BASH-deficient mice: limited primary repertoire and antibody formation, but sufficient affinity maturation and memory B cell generation, in anti-NP response.Int Immunol. 2004 Aug;16(8):1161-71. doi: 10.1093/intimm/dxh116. Epub 2004 Jul 5. Int Immunol. 2004. PMID: 15237108
-
Low-affinity IgM antibodies lacking somatic hypermutations are produced in the secondary response of C57BL/6 mice to (4-hydroxy-3-nitrophenyl)acetyl hapten.Int Immunol. 2014 Apr;26(4):195-208. doi: 10.1093/intimm/dxt057. Epub 2013 Nov 27. Int Immunol. 2014. PMID: 24285827
-
Regional differences of B cell immune responses to (4-hydroxy-3 nitrophenylacetyl) acetic acid (NP) antigen in senescent mucosal immune system.Reg Immunol. 1992 Nov-Dec;4(6):381-90. Reg Immunol. 1992. PMID: 1297408
-
Heterogeneous and monoclonal helper T cells induce similar anti-(4-hydroxy-3-nitrophenyl)acetyl (NP) antibody populations in the primary adoptive response. I. Isotype distribution.Eur J Immunol. 1984 Feb;14(2):188-94. doi: 10.1002/eji.1830140215. Eur J Immunol. 1984. PMID: 6199216
-
Isotype-specific selection of high affinity memory B cells in nasal-associated lymphoid tissue.J Exp Med. 2001 Dec 3;194(11):1597-607. doi: 10.1084/jem.194.11.1597. J Exp Med. 2001. PMID: 11733574 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Miscellaneous

