Quantification of alcohol intake in patients with steatotic liver disease and excessive alcohol intake
- PMID: 39698234
- PMCID: PMC11652777
- DOI: 10.1016/j.jhepr.2024.101200
Quantification of alcohol intake in patients with steatotic liver disease and excessive alcohol intake
Erratum in
-
Erratum regarding previously published articles.JHEP Rep. 2025 Feb 17;7(3):101359. doi: 10.1016/j.jhepr.2025.101359. eCollection 2025 Mar. JHEP Rep. 2025. PMID: 40170909 Free PMC article.
Abstract
Background & aims: Quantifying alcohol intake is crucial for subclassifying participants with steatotic liver disease (SLD) and interpreting clinical trials of alcohol-related liver disease (ALD) and metabolic and alcohol-related liver disease (MetALD). However, the accuracy of self-reported alcohol intake is considered imprecise. We compared the diagnostic and prognostic utility of self-reported alcohol intake with blood-based biomarkers of alcohol intake: phosphatidylethanol (PEth) and carbohydrate-deficient transferrin (CDT).
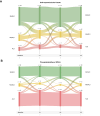
Methods: We studied 192 participants from two randomized controlled trials on MetALD and ALD, all with current or former excessive alcohol intake (≥24/36 [♀/♂] grams daily for at least 1 year) and biopsy-proven liver disease. We assessed self-reported alcohol intake, PEth, and CDT at four time points. We collected follow-up data on hepatic decompensation and death manually through electronic medical records.
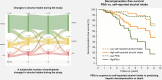
Results: Most participants were male (n = 161, 84%) with a mean age of 59 (SD 9) years and 73 participants reported 1-week abstinence before inclusion; the remaining reported a median alcohol intake of 43 g/day. Median PEth was 0.5 μmol/L (IQR: 0.0-1.3) and %CDT = 1.9 (IQR: 1.6-2.3). Of 32 patients reporting at least 6 months of abstinence; 27 (84%) was confirmed by PEth <0.05 μmol/L. Self-reported alcohol intake correlated well with PEth (r = 0.617) and moderately with CDT (r = 0.316). Self-reported alcohol intake, PEth, and CDT all predicted hepatic decompensation and death. However, PEth showed the highest prediction, surpassing self-reported alcohol intake (Harrel's C, PEth = 0.80 vs. self-reported = 0.68, p = 0.026).
Conclusions: Self-reported abstinence can be considered reliable in clinical trials. However, PEth is superior in predicting hepatic decompensation and death in patients with MetALD and ALD.
Impact and implications: An accurate quantification of alcohol intake is crucial in the clinical phenotyping of patients with steatotic liver disease and when designing clinical trials. This study found self-reported abstinence to be reliable but phosphatidylethanol was a more accurate prognostic biomarker of hepatic decompensation and death in a clinical trial setting. Findings may inform the design of future trials in patients with steatotic liver disease.
Keywords: alcohol use disorder; alcohol-related liver disease; biomarker; cirrhosis; steatotic liver disease.
© 2024 The Author(s).
Conflict of interest statement
JKH has received a speaking fee from Norgine. KPL has received a speaking fee, support for travels from Siemens, and is a co-founder and board member for Evido. ET has received speaking fees from Echosens, NovoNordisk, Orphalan, and Dr Falk and participated in advisory boards for Boehringer, NovoNordisk, Pfizer, Orphalan, Univar, Alexion, and Siemens Healthineers. JT has received speaking and/or consulting fees from Versantis, Gore, Boehringer-Ingelheim, Falk, Grifols, Genfit, and CSL Behring. MT has received speaking fees from Siemens Healthcare, Norgine, Echosens, Tillotts pharma, Takeda and Madrigal; consulting fees from GE Healthcare, Boehringer Ingelheim and GSK, is vice chair on the board of Alcohol & Society (NGO) and co-founder and board member for Evido. AK has served as speaker for Novo Nordisk, Norgine, Siemens, and Nordic Bioscience and participated in advisory boards for Norgine, Siemens, Resalis Therapeutics, Boehringer Ingelheim, and Novo Nordisk. Research support: Norgine, Siemens, Nordic Bioscience, AstraZeneca, Echosens. Board member and co-founder Evido. EDH, NT, SJ, MLB, CDH, SD, PA, IV, KB, KT, GHJ, and TB declare no conflicts of interest. Please refer to the accompanying ICMJE disclosure forms for further details.
Figures



References
-
- Asrani S.K., Devarbhavi H., Eaton J., et al. Burden of liver diseases in the world. J Hepatol. 2019;70:151–171. - PubMed
-
- Rinella M.E., Lazarus J.V., Ratziu V., et al. A multi-society Delphi consensus statement on new fatty liver disease nomenclature. J Hepatol. 2023;78:1966–1986.
-
- Israelsen M., Torp N., Johansen S., et al. MetALD: new opportunities to understand the role of alcohol in steatotic liver disease. Lancet Gastroenterol Hepatol. 2023;8:866–868. - PubMed
-
- Israelsen M., Torp N., Johansen S., et al. Validation of the new nomenclature of steatotic liver disease in patients with a history of excessive alcohol intake: an analysis of data from a prospective cohort study. Lancet Gastroenterol Hepatol. 2024;9:218–228. - PubMed
-
- Ginès P., Graupera I., Lammert F., et al. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol. 2016;1:256–260. - PubMed
LinkOut - more resources
Full Text Sources

