Hijacking intercellular trafficking for the spread of protein aggregates in neurodegenerative diseases: a focus on tunneling nanotubes (TNTs)
- PMID: 39698299
- PMCID: PMC11648486
- DOI: 10.20517/evcna.2023.05
Hijacking intercellular trafficking for the spread of protein aggregates in neurodegenerative diseases: a focus on tunneling nanotubes (TNTs)
Abstract
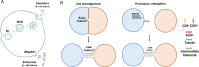
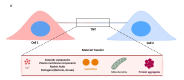
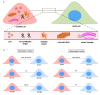
Over the years, the influence of secretory mechanisms on intercellular communication has been extensively studied. In the central nervous system (CNS), both trans-synaptic (neurotransmitter-based) and long-distance (extracellular vesicles-based) communications regulate activities and homeostasis. In less than a couple of decades, however, there has been a major paradigm shift in our understanding of intercellular communication. Increasing evidence suggests that besides secretory mechanisms (via extracellular vesicles), several cells are capable of establishing long-distance communication routes referred to as Tunneling Nanotubes (TNTs). TNTs are membranous bridges classically supported by F-Actin filaments, allowing for the exchange of different types of intracellular components between the connected cells, ranging from ions and organelles to pathogens and toxic protein aggregates. The roles of TNTs in pathological spreading of several neurodegenerative conditions such as Prion diseases, Alzheimer's disease (AD), Parkinson's disease (PD), and Huntington's disease (HD) have been well established. However, the fragile nature of these structures and lack of specific biomarkers raised some skepticism regarding their existence. In this review, we will first place TNTs within the spectrum of intercellular communication mechanisms before discussing their known and hypothesized biological relevance in vitro and in vivo in physiological and neurodegenerative contexts. Finally, we discuss the challenges and promising prospects in the field of TNT studies.
Keywords: Tunneling nanotubes; intercellular communication; neurodegenerative diseases.
© The Author(s) 2023.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures
References
Publication types
LinkOut - more resources
Full Text Sources
