Virus nanotechnology for intratumoural immunotherapy
- PMID: 39698315
- PMCID: PMC11655125
- DOI: 10.1038/s44222-024-00231-z
Virus nanotechnology for intratumoural immunotherapy
Abstract
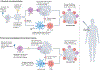
Viruses can be designed to be tools and carrier vehicles for intratumoural immunotherapy. Their nanometre-scale size and shape allow for functionalization with or encapsulation of medical cargoes and tissue-specific ligands. Importantly, immunotherapies may particularly benefit from the inherent immunomodulatory properties of viruses. For example, mammalian viruses have already been tested for oncolytic virotherapy, and bacteriophages and plant viruses can be engineered for immunotherapeutic treatment approaches. In this Review, we discuss how viruses - including oncolytic viruses, immunomodulatory plant viruses and bacteriophages - and virus-like particles can be designed for intratumoural immunotherapy to elicit anti-tumour immunity and induce systemic anti-tumour responses at distant non-injected sites. We further highlight the engineering of viruses and virus-like particles as drug-delivery systems, and outline key translational challenges and clinical opportunities.
Conflict of interest statement
Competing interests The authors declare the following competing financial interest(s): N.F.S. and S.N.F. are co-founders of, have equity in, and have a financial interest with Mosaic ImmunoEngineering Inc. N.F.S. is a co-founder of, and serves as manager of, Pokometz Scientific LLC, under which she is a paid consultant to Flagship Labs 95 Inc. and Arana Biosciences Inc. M.S. is a consultant of and has equity in Mosaic ImmunoEngineering Inc. The other authors declare no potential conflicts of interest.
Figures

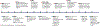



Similar articles
-
Oncolytic Newcastle disease virus expressing a checkpoint inhibitor as a radioenhancing agent for murine melanoma.EBioMedicine. 2019 Nov;49:96-105. doi: 10.1016/j.ebiom.2019.10.032. Epub 2019 Oct 29. EBioMedicine. 2019. PMID: 31676387 Free PMC article.
-
Intratumoural administration and tumour tissue targeting of cancer immunotherapies.Nat Rev Clin Oncol. 2021 Sep;18(9):558-576. doi: 10.1038/s41571-021-00507-y. Epub 2021 May 18. Nat Rev Clin Oncol. 2021. PMID: 34006998 Free PMC article. Review.
-
Development of a new fusion-enhanced oncolytic immunotherapy platform based on herpes simplex virus type 1.J Immunother Cancer. 2019 Aug 10;7(1):214. doi: 10.1186/s40425-019-0682-1. J Immunother Cancer. 2019. PMID: 31399043 Free PMC article.
-
Plant Viruses and Bacteriophage-Based Reagents for Diagnosis and Therapy.Annu Rev Virol. 2020 Sep 29;7(1):559-587. doi: 10.1146/annurev-virology-010720-052252. Annu Rev Virol. 2020. PMID: 32991265 Free PMC article. Review.
-
Engineering Newcastle Disease Virus as an Oncolytic Vector for Intratumoral Delivery of Immune Checkpoint Inhibitors and Immunocytokines.J Virol. 2020 Jan 17;94(3):e01677-19. doi: 10.1128/JVI.01677-19. Print 2020 Jan 17. J Virol. 2020. PMID: 31694938 Free PMC article.
Cited by
-
Preclinical SC and IV repeat-dose toxicology of a cowpea mosaic virus - A cancer immunotherapy candidate.Toxicol Rep. 2025 Apr 7;14:102022. doi: 10.1016/j.toxrep.2025.102022. eCollection 2025 Jun. Toxicol Rep. 2025. PMID: 40276251 Free PMC article.
-
Tumor-microenvironment triggered Mn-Gd based nanosystem for breast carcinoma suppression via synergistic radiotherapy and glutathione-depleting along with glucose oxidase combination enhanced Ros storm.J Nanobiotechnology. 2025 Aug 11;23(1):557. doi: 10.1186/s12951-025-03636-z. J Nanobiotechnology. 2025. PMID: 40790599 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources
