SGLT2-Inhibition in Patients With Alport Syndrome
- PMID: 39698346
- PMCID: PMC11652101
- DOI: 10.1016/j.ekir.2024.09.014
SGLT2-Inhibition in Patients With Alport Syndrome
Abstract
Introduction: Large-scale trials showed positive outcomes of sodium-glucose cotransporter-2 inhibitors (SGLT2i) in adults with chronic kidney disease (CKD). Whether the use of SGLT2i is safe and effective in patients with the common hereditary CKD Alport syndrome (AS) has not yet been investigated specifically in larger cohorts.
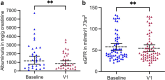
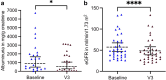
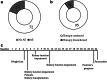
Methods: This observational, multicenter, international study (NCT02378805) assessed 112 patients with AS after start of SGLT2i. The study's primary end point was change of albuminuria in albumin/g creatinine from the start of therapy.
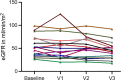
Results: Compared to randomized trials investigating the effect of SGLT2i in CKD, the adult patients in this study were younger (aged 38 ± 14 years) and had a better estimated glomerular filtration rate (eGFR, 63 ± 35 ml/min per 1.73 m2; n = 98). Maximum follow-up was 32 months. Compared to baseline, at the first 3 follow-up visits (months 1 to 3, 4 to 8, and 9 to 15) after initiation of SGLT2i therapy, a significant reduction of albuminuria in mg albumin/g creatinine (>30%) was observed. Mean loss of eGFR was 9 ± 12 ml/min per 1.73 m2 almost 1 year after initiation of SGLT2i therapy (n = 35). At a total of 71 patient-years at risk, 0.24 adverse events (AEs) per patient-year on SGLT2i were reported.
Conclusion: This study indicates that, additive to renin-angiotensin system (RAS)-inhibition (RASi), SGLT2i have the potential to reduce the amount of albuminuria in patients with AS. Future studies are needed to investigate the long-term effects of SGLT2i on CKD progression in patients with AS to assess whether the observed reduction in albuminuria translates to a delay in kidney failure (KF).
Keywords: Alport syndrome; COL4; albuminuria; dapagliflozin; empagliflozin; kidney failure.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

