Anti Phospholipase A2 Receptor 1 Antibodies and Membranous Nephropathy Recurrence After Kidney Transplantation
- PMID: 39698349
- PMCID: PMC11652070
- DOI: 10.1016/j.ekir.2024.09.012
Anti Phospholipase A2 Receptor 1 Antibodies and Membranous Nephropathy Recurrence After Kidney Transplantation
Abstract
Introduction: Membranous nephropathy can lead to end-stage kidney disease, for which kidney transplantation is the preferred therapy. However, the disease often relapses, which can impact allograft survival.
Methods: We conducted a prospective multicenter study in France involving 72 patients with membranous nephropathy who were awaiting and then underwent kidney transplantation. In addition, we established a retrospective validation cohort of 65 patients. The primary objective was to evaluate the prognostic significance of pretransplant anti phospholipase A2 receptor 1 (PLA2R1) antibodies on the recurrence of membranous nephropathy. The study also assessed the incidence rate, time to onset, and risk factors for recurrence, as well as allograft outcome.
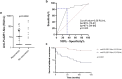
Results: The prospective cohort showed a 26% cumulative incidence of membranous nephropathy recurrence after a median follow-up of 23.5 months. This was confirmed by a 28% cumulative incidence after a median follow-up of 67 months in the retrospective cohort. A strong association was found between the presence of anti-PLA2R1 antibodies prior to transplantation and the risk of disease recurrence (risk ratio = 5.9; 95% confidence interval [CI]: 2.3-15.7; P < 0.0001). These results were confirmed in the retrospective cohort. Monitoring of anti-PLA2R1 antibodies in the immediate posttransplant period is of limited value, because recurrence occurred early in the first 6 months (median delay of 5 [3-14] months) after transplantation despite decreasing antibody levels.
Conclusion: The presence of anti-PLA2R1 antibodies prior to transplantation was a strong predictor of recurrence of allograft membranous nephropathy. An individualized immunomonitoring and management strategy for kidney transplant candidates with anti-PLA2R1-associated membranous nephropathy should be considered.
Keywords: anti-PLA2R1 antibodies; kidney transplantation; membranous nephropathy; recurrence.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures


References
LinkOut - more resources
Full Text Sources

