Combination Therapy With Rituximab and Low-Dose Cyclophosphamide and Prednisone in Membranous Nephropathy
- PMID: 39698354
- PMCID: PMC11652067
- DOI: 10.1016/j.ekir.2024.08.033
Combination Therapy With Rituximab and Low-Dose Cyclophosphamide and Prednisone in Membranous Nephropathy
Abstract
Introduction: Standard treatment with cyclophosphamide (CP) or rituximab (RTX) is suboptimal. We adapted and used the low-dose regimen used in vasculitis (RTX 2 × 1000 mg, CP 1.5 mg/kg/d × 8 weeks, and prednisone [i.v. 2 × 1 g + 3 weeks oral starting at 1 mg/kg]).
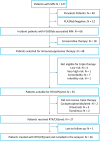
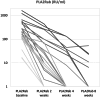
Methods: High-risk, anti-PLA2R antibodies (PLA2Rab)-positive patients with membranous nephropathy (MN) were included in this single-arm prospective cohort study. PLA2Rab levels were regularly measured. We report the PLA2Rab kinetics and overall immunological and clinical remission (CR) rate.
Results: We analyzed 26 patients (15 males, aged 57 ± 14 years, PLA2Rab titer 176 [115-460] RU/ml, serum creatinine 128 [102-136] μmol/l, serum albumin 18 [14-21] g/l, and urinary protein-to-creatinine ratio [uPCR] 7.1 [5.7-10] g/10 mmol). Within 8 weeks immunological remission (IR) (enzyme-linked immunosorbent assay < 14 RU/ml) was 88 %. Proteinuria remission after initial therapy developed in 21 patients. Seven patients received renewed therapy, which resulted in proteinuria remission in all. IR and CR were associated with baseline PLA2Rab tertile. Five of 7 patients in need of additional therapy were identified at 4 weeks after start of therapy by PLA2Rab half-life (T1/2) > 7 days. Serious adverse events occurred in 4 patients. Adverse events were mild; leukopenia was most frequent.
Conclusion: Low-dose triple therapy induced a rapid IR and CR in most patients. Patients with insufficient clinical response were characterized by high baseline PLA2Rab levels and longer PLA2Rab T1/2. Assessment of PLA2Rab levels within 2 to 4 weeks after start of therapy may enable to identify patients who need more intensive therapy.
Keywords: anti-PLA2R antibodies; immunosuppression; membranous nephropathy.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures



References
LinkOut - more resources
Full Text Sources
Miscellaneous

