Pilot Trial of Hydroxychloroquine as Add-On Therapy in Patients With Membranous Nephropathy
- PMID: 39698370
- PMCID: PMC11652102
- DOI: 10.1016/j.ekir.2024.09.016
Pilot Trial of Hydroxychloroquine as Add-On Therapy in Patients With Membranous Nephropathy
Abstract
Introduction: Kidney Disease Improving Global Outcomes guidelines indicate that glucocorticoids and immunosuppressants comprise the first therapeutic regimens after 4 to 6 months of treatment for high-risk primary membranous nephropathy (PMN). However, some patients cannot achieve complete or partial remission at 6 months. This study aimed to evaluate the efficacy of traditional immunotherapy combined with hydroxychloroquine (HCQ), a well-known immune regulator, in patients with PMN.
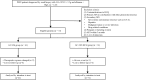
Methods: This was a single-center, open-label, prospective study. We recruited 72 patients with nephrotic syndrome and PMN proven by renal biopsy from May 2020 to June 2024. We compared changes in proteinuria, serum albumin levels, estimated glomerular filtration rate (eGFR), and relapse rate at 3, 6, 9, and 12 months follow-up in 41 patients who received glucocorticoid and immunosuppressant, and in 31 who received HCQ plus standard-of-care.
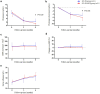
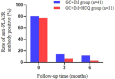
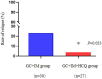
Results: Baseline characteristics showed no statistical significance between the 2 groups. However, the HCQ group showed significantly reduced proteinuria compared to standard-of-care group. A reduced proteinuria was seen at 6 months (1.2 [0.4-2.2] vs. 2.2 [1.0-3.8] g/d, P = 0.029) and the relapse rate with 12 months follow-up was also significantly decreased in the HCQ group compared to the standard-of-care group (3.7% vs. 23.3%, P = 0.033).
Conclusions: HCQ may serve as an effective add-on therapy for PMN.
Keywords: add-on therapy; glucocorticoid; hydroxychloroquine; immunosuppressants; primary membranous nephropathy.
© 2024 International Society of Nephrology. Published by Elsevier Inc.
Figures

References
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

