Platform technologies and human cell lines for the production of therapeutic exosomes
- PMID: 39698504
- PMCID: PMC11648496
- DOI: 10.20517/evcna.2020.01
Platform technologies and human cell lines for the production of therapeutic exosomes
Abstract
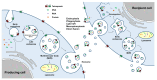
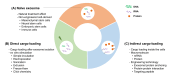
Exosomes are extracellular vesicles secreted by most cell types and represent various biological properties depending on their producing cells. They are also known to be important mediators of intercellular communication. Recent data suggest that exosomes can mediate the therapeutic effects of their parental cells; hence, they have been in the spotlight as novel therapeutics. To develop and manufacture effective therapeutic exosomes, customized strategies are needed to use appropriate technologies for exosome engineering and to select suitable production cell lines. In this review, we provide an overview of currently available exosome engineering platform technologies for loading active pharmaceutical ingredient cargo and the types of human cells/cell lines that are being used as exosome-producing cells, particularly focusing on their characteristics, advantages, and disadvantages.
Keywords: HEK293; Therapeutic exosome; dendritic cells; exosome engineering technologies; human cells; stem cells.
© The Author(s) 2021.
Conflict of interest statement
Choi C, the founder and shareholder of ILIAS Biologics Inc., is an inventor of a patent related to “EXPLOR®” technology filed by ILIAS Biologics Inc. (no. KR 10-1877010 and US 10,702,581).
Figures
Similar articles
-
The emerging role of exosomes as novel therapeutics: Biology, technologies, clinical applications, and the next.Am J Reprod Immunol. 2021 Feb;85(2):e13329. doi: 10.1111/aji.13329. Epub 2020 Sep 12. Am J Reprod Immunol. 2021. PMID: 32846024 Free PMC article. Review.
-
A comprehensive review on recent advances in exosome isolation and characterization: Toward clinical applications.Transl Oncol. 2024 Dec;50:102121. doi: 10.1016/j.tranon.2024.102121. Epub 2024 Sep 14. Transl Oncol. 2024. PMID: 39278189 Free PMC article. Review.
-
Optimization of exosome-based cell-free strategies to enhance endogenous cell functions in tissue regeneration.Acta Biomater. 2023 Nov;171:68-84. doi: 10.1016/j.actbio.2023.09.023. Epub 2023 Sep 18. Acta Biomater. 2023. PMID: 37730080 Review.
-
Manufacturing Therapeutic Exosomes: from Bench to Industry.Mol Cells. 2022 May 31;45(5):284-290. doi: 10.14348/molcells.2022.2033. Mol Cells. 2022. PMID: 35534190 Free PMC article. Review.
-
Exosome theranostics: Comparative analysis of P body and exosome proteins and their mutations for clinical applications.Funct Integr Genomics. 2024 Jul 12;24(4):124. doi: 10.1007/s10142-024-01404-0. Funct Integr Genomics. 2024. PMID: 38995459 Review.
Cited by
-
Quantifying extracellular vesicle heterogeneity: the effect of process conditions on protein cargo for skin therapy.Stem Cell Res Ther. 2025 May 4;16(1):224. doi: 10.1186/s13287-025-04279-5. Stem Cell Res Ther. 2025. PMID: 40320543 Free PMC article. Review.
-
Exosomal Bupivacaine: Integrating Nerve Barrier Penetration Capability and Sustained Drug Release for Enhanced Potency in Peripheral Nerve Block and Reduced Toxicity.Adv Funct Mater. 2024 Oct 15;34(42):2406876. doi: 10.1002/adfm.202406876. Epub 2024 Jul 20. Adv Funct Mater. 2024. PMID: 40027274
-
Effect of Small Extracellular Vesicles Produced by Mesenchymal Stem Cells on 5xFAD Mice Hippocampal Cultures.Int J Mol Sci. 2025 Apr 24;26(9):4026. doi: 10.3390/ijms26094026. Int J Mol Sci. 2025. PMID: 40362265 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
