Cargo exchange between human and bacterial extracellular vesicles in gestational tissues: a new paradigm in communication and immune development
- PMID: 39698538
- PMCID: PMC11648491
- DOI: 10.20517/evcna.2024.21
Cargo exchange between human and bacterial extracellular vesicles in gestational tissues: a new paradigm in communication and immune development
Abstract
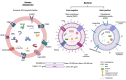
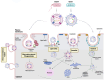
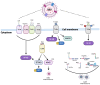
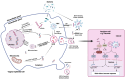
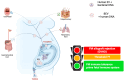
Host-bacteria and bacteria-bacteria interactions can be facilitated by extracellular vesicles (EVs) secreted by both human and bacterial cells. Human and bacterial EVs (BEVs) propagate and transfer immunogenic cargos that may elicit immune responses in nearby or distant recipient cells/tissues. Hence, direct colonization of tissues by bacterial cells is not required for immunogenic stimulation. This phenomenon is important in the feto-maternal interface, where optimum tolerance between the mother and fetus is required for a successful pregnancy. Though the intrauterine cavity is widely considered sterile, BEVs from diverse sources have been identified in the placenta and amniotic cavity. These BEVs can be internalized by human cells, which may help them evade host immune surveillance. Though it appears logical, whether bacterial cells internalize human EVs or human EV cargo is yet to be determined. However, the presence of BEVs in placental tissues or amniotic cavity is believed to trigger a low-grade immune response that primes the fetal immune system for ex-utero survival, but is insufficient to disrupt the progression of pregnancy or cause immune intolerance required for adverse pregnancy events. Nevertheless, the exchange of bioactive cargos between human and BEVs, and the mechanical underpinnings and health implications of such interactions, especially during pregnancy, are still understudied. Therefore, while focusing on the feto-maternal interface, we discussed how human cells take up BEVs and whether bacterial cells take up human EVs or their cargo, the exchange of cargos between human and BEVs, host cell (feto-maternal) inflammatory responses to BEV immunogenic stimulation, and associations of these interactions with fetal immune priming and adverse reproductive outcomes such as preeclampsia and preterm birth.
Keywords: Extracellular vesicles; fetal membranes; feto-maternal interface; immune priming; outer membrane vesicles; placenta.
© The Author(s) 2024.
Conflict of interest statement
All authors declared that there are no conflicts of interest.
Figures
References
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
