G protein-coupled receptors: a gateway to targeting oncogenic EVs?
- PMID: 39698539
- PMCID: PMC11648488
- DOI: 10.20517/evcna.2024.10
G protein-coupled receptors: a gateway to targeting oncogenic EVs?
Abstract
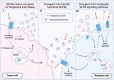
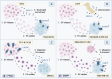
Dysregulated intercellular communication is a key feature driving cancer progression. Recently, extracellular vesicles (EVs) have added a new channel to this dense communication network. Despite solid evidence that EVs are central mediators of dysregulated signaling in onco-pathological settings, this has yet to be translated into clinically actionable strategies. The heterogeneity of EV cargo molecules, plasticity of biogenesis routes, and large overlap with their role in physiological communication, complicate a potential targeting strategy. However, recent work has linked EV biology to perhaps the "most druggable" proteins - G protein-coupled receptors (GPCRs). GPCR targeting accounts for ~60% of drugs in development and more than a third of all currently approved drugs, spanning almost all areas of medicine. Although several GPCRs have been linked to cancer initiation and progression, relatively few agents have made it into oncological regimes, suggesting that their potential is underexploited. Herein, we examine the molecular mechanisms linking GPCRs to EV communication in cancer settings. We propose that GPCRs hold potential in the search for EV-targeting in oncology.
Keywords: G protein-coupled receptors (GPCRs); extracellular vesicles (EVs); oncogenic signaling; therapeutic targeting.
© The Author(s) 2024.
Conflict of interest statement
All authors declare that there are no conflicts of interest.
Figures

References
Publication types
LinkOut - more resources
Full Text Sources
