The causal effects of gut microbiota on quantitative susceptibility mapping (QSM) and T2* imaging-derived phenotypes: insights from a Mendelian randomization study
- PMID: 39698716
- PMCID: PMC11652028
- DOI: 10.21037/qims-24-318
The causal effects of gut microbiota on quantitative susceptibility mapping (QSM) and T2* imaging-derived phenotypes: insights from a Mendelian randomization study
Abstract
Background: Gut microbiota are associated with brain imaging-derived phenotypes (IDPs); however, the specific causal relationship between the gut microbiota and brain iron-related IDPs remains unclear. Thus, we sought to analyze the potential causal effects of gut microbiota on brain iron-related IDPs using Mendelian randomization (MR).
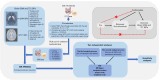
Methods: We obtained the data of 196 gut microbiota from a genome-wide association study (GWAS) from the MiBioGen database, as well as the data of 18 quantitative susceptibility mapping (QSM) IDPs and 10 T2* IDPs from the United Kingdom Biobank (UKB). We then conducted one-way two-sample MR analyses to examine their causal interactions. To guarantee the robustness of the results, we performed two independent analysis processes by selecting statistically significant instrumental variables (IVs) with a distinct level of statistical strictness, and derived the intersection of these two analyses.
Results: Our results showed that the genus Howardella was positively correlated with the median susceptibility in the right caudate [β: 0.0935, 95% confidence interval (CI): 0.0601, 0.1269, Pinverse variance weighting (IVW)=4.00E-08]; the genus Dialister was positively correlated with the median susceptibility in the right accumbens (β: 0.0949, 95% CI: 0.0575, 0.1324, PIVW=6.90E-07); the genus Butyricicoccus was positively associated with the median T2* in the left hippocampus with the additional deconfounding of the background field gradient (β: 0.1543, 95% CI: 0.0959, 0.2127, PIVW=2.20E-07); the genus Desulfovibrio was positively related to the T2* white matter hyperintensity (WMH) IDP with WMH volume regressed out (β: 0.1168, 95% CI: 0.0697, 0.1639, PIVW=1.20E-06). Notably, both the family Defluviitaleaceae (β: -0.1215, 95% CI: -0.1604, -0.0827, PIVW=8.40E-10) and genus DefluviitaleaceaeUCG011 (β: -0.1142, 95% CI: -0.1614, -0.0670, PIVW=2.10E-06) were negatively correlated with the median T2* in the right accumbens with the additional deconfounding of the background field gradient.
Conclusions: This study found genetic evidence that gut microbiota dysbiosis has causal effects on brain iron-related IDPs. Our findings provide novel insights into the diagnosis and therapeutic management of central nervous system (CNS) diseases.
Keywords: Gut microbiota; Mendelian randomization (MR); T2* mapping; imaging-derived phenotypes (IDPs); quantitative susceptibility mapping (QSM).
2024 AME Publishing Company. All rights reserved.
Conflict of interest statement
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://qims.amegroups.com/article/view/10.21037/qims-24-318/coif). L.Y. reports that this work was supported by the Fundamental Research Funds for the Central Universities of Central South University (No. 1053320230244). S.G. is a current employee of Infervision Medical Technology Co., Ltd. J.L. reports that this work was supported by the National Natural Science Foundation of China (No. U22A20303), the Innovative Province Special Construction foundation of Hunan Province (No. 2019SK2131), the Science and Technology Innovation Program of Hunan province (No. 2021RC4016), and the Clinical Research Center for Medical Imaging in Hunan Province in China (No. 2020SK4001). The other authors have no conflicts of interest to declare.
Figures

References
LinkOut - more resources
Full Text Sources
