RNA elements required for the high efficiency of West Nile virus-induced ribosomal frameshifting
- PMID: 39698810
- PMCID: PMC11797035
- DOI: 10.1093/nar/gkae1248
RNA elements required for the high efficiency of West Nile virus-induced ribosomal frameshifting
Abstract
West Nile virus (WNV) requires programmed -1 ribosomal frameshifting for translation of the viral genome. The efficiency of WNV frameshifting is among the highest known. However, it remains unclear why WNV exhibits such a high frameshifting efficiency. Here, we employed dual-luciferase reporter assays in multiple human cell lines to probe the RNA requirements for highly efficient frameshifting by the WNV genome. We find that both the sequence and structure of a predicted RNA pseudoknot downstream of the slippery sequence-the codons in the genome on which frameshifting occurs-are required for efficient frameshifting. We also show that multiple proposed RNA secondary structures downstream of the slippery sequence are inconsistent with efficient frameshifting. We also find that the base of the pseudoknot structure likely is unfolded prior to frameshifting. Finally, we show that many mutations in the WNV slippery sequence allow efficient frameshifting, but often result in aberrant shifting into other reading frames. Mutations in the slippery sequence also support a model in which frameshifting occurs concurrent with or after ribosome translocation. These results provide a comprehensive analysis of the molecular determinants of WNV-programmed ribosomal frameshifting and provide a foundation for the development of new antiviral strategies targeting viral gene expression.
© The Author(s) 2024. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures
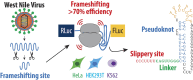




Update of
-
RNA elements required for the high efficiency of West Nile Virus-induced ribosomal frameshifting.bioRxiv [Preprint]. 2024 Oct 16:2024.10.16.618579. doi: 10.1101/2024.10.16.618579. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Jan 24;53(3):gkae1248. doi: 10.1093/nar/gkae1248. PMID: 39464146 Free PMC article. Updated. Preprint.
Similar articles
-
RNA elements required for the high efficiency of West Nile Virus-induced ribosomal frameshifting.bioRxiv [Preprint]. 2024 Oct 16:2024.10.16.618579. doi: 10.1101/2024.10.16.618579. bioRxiv. 2024. Update in: Nucleic Acids Res. 2025 Jan 24;53(3):gkae1248. doi: 10.1093/nar/gkae1248. PMID: 39464146 Free PMC article. Updated. Preprint.
-
Structural and Functional Characterization of Programmed Ribosomal Frameshift Signals in West Nile Virus Strains Reveals High Structural Plasticity Among cis-Acting RNA Elements.J Biol Chem. 2016 Jul 22;291(30):15788-95. doi: 10.1074/jbc.M116.735613. Epub 2016 May 23. J Biol Chem. 2016. PMID: 27226636 Free PMC article.
-
Secondary structure and mutational analysis of the ribosomal frameshift signal of rous sarcoma virus.J Mol Biol. 1998 Nov 27;284(2):205-25. doi: 10.1006/jmbi.1998.2186. J Mol Biol. 1998. PMID: 9813113 Free PMC article.
-
Structure, stability and function of RNA pseudoknots involved in stimulating ribosomal frameshifting.J Mol Biol. 2000 Apr 28;298(2):167-85. doi: 10.1006/jmbi.2000.3668. J Mol Biol. 2000. PMID: 10764589 Free PMC article. Review.
-
A review on architecture of the gag-pol ribosomal frameshifting RNA in human immunodeficiency virus: a variability survey of virus genotypes.J Biomol Struct Dyn. 2017 Jun;35(8):1629-1653. doi: 10.1080/07391102.2016.1194231. Epub 2016 Aug 2. J Biomol Struct Dyn. 2017. PMID: 27485859 Review.
Cited by
-
Human endometrial stem cell-derived small extracellular vesicles enhance neurite outgrowth and peripheral nerve regeneration through activating the PI3K/AKT signaling pathway.J Transl Med. 2025 Jan 3;23(1):6. doi: 10.1186/s12967-024-06048-z. J Transl Med. 2025. PMID: 39754260 Free PMC article.
References
-
- Smithburn K.C., Hughes T.P., Burke A.W., Paul J.H.. A neurotropic virus isolated from the blood of a native of Uganda. Am. J. Trop. Med. 1940; s1-20:471–492.

