A subset of human dermal fibroblasts overexpressing Cockayne syndrome group B protein resist UVB radiation-mediated premature senescence
- PMID: 39698891
- PMCID: PMC11896172
- DOI: 10.1111/acel.14422
A subset of human dermal fibroblasts overexpressing Cockayne syndrome group B protein resist UVB radiation-mediated premature senescence
Abstract
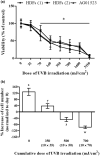
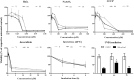
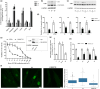
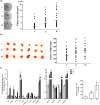
Ultraviolet B (UVB) radiation is a major contributor to skin photoaging. Although mainly absorbed by the epidermis, UVB photons managing to penetrate the upper dermis affect human dermal fibroblasts (HDFs), leading, among others, to the accumulation of senescent cells. In vitro studies have shown that repeated exposures to subcytotoxic UVB radiation doses provoke HDFs' premature senescence shortly after the end of the treatment period. Here, we found that repetitive exposures to non-cytotoxic UVB radiation doses after several days lead to mixed cultures, containing both senescent cells and fibroblasts resisting senescence. "Resistant" fibroblasts were more resilient to a novel intense UVB radiation stimulus. RNA-seq analysis revealed that ERCC6, encoding Cockayne syndrome group B (CSB) protein, is up-regulated in resistant HDFs compared to young and senescent cells. CSB was found to be a key molecule conferring protection toward UVB-induced cytotoxicity and senescence, as siRNA-mediated CSB loss-of-expression rendered HDFs significantly more susceptible to a high UVB radiation dose, while cells from a CSB-deficient patient were found to be more sensitive to UVB-mediated toxicity, as well as senescence. UVB-resistant HDFs remained normal (able to undergo replicative senescence) and non-tumorigenic. Even though they formed a distinct population in-between young and senescent cells, resistant HDFs retained numerous tissue-impairing characteristics of the senescence-associated secretory phenotype, including increased matrix metalloprotease activity and promotion of epidermoid tumor xenografts in immunodeficient mice. Collectively, here we describe a novel subpopulation of HDFs showing increased resistance to UVB-mediated premature senescence while retaining undesirable traits that may negatively affect skin homeostasis.
Keywords: CSB; RNA‐seq; UVB; human dermal fibroblasts; photoaging; resistance; senescence.
© 2024 The Author(s). Aging Cell published by Anatomical Society and John Wiley & Sons Ltd.
Conflict of interest statement
None declared.
Figures


Similar articles
-
Molecular mechanisms of UVB-induced senescence of dermal fibroblasts and its relevance for photoaging of the human skin.Exp Gerontol. 2017 Aug;94:78-82. doi: 10.1016/j.exger.2017.01.009. Epub 2017 Jan 14. Exp Gerontol. 2017. PMID: 28093316
-
UVB-induced premature senescence of human diploid skin fibroblasts.Int J Biochem Cell Biol. 2002 Nov;34(11):1331-9. doi: 10.1016/s1357-2725(02)00022-5. Int J Biochem Cell Biol. 2002. PMID: 12200029
-
Enhanced SIRT1 Activity by Galangin Mitigates UVB-Induced Senescence in Dermal Fibroblasts via p53 Acetylation Regulation and Activation.J Agric Food Chem. 2024 Oct 23;72(42):23286-23294. doi: 10.1021/acs.jafc.4c05945. Epub 2024 Oct 14. J Agric Food Chem. 2024. PMID: 39401943
-
Cockayne Syndrome Group B (CSB): The Regulatory Framework Governing the Multifunctional Protein and Its Plausible Role in Cancer.Cells. 2021 Apr 10;10(4):866. doi: 10.3390/cells10040866. Cells. 2021. PMID: 33920220 Free PMC article. Review.
-
Perspectives in the investigation of Cockayne syndrome group B neurological disease: the utility of patient-derived brain organoid models.J Zhejiang Univ Sci B. 2024 Oct 2;25(10):878-889. doi: 10.1631/jzus.B2300712. J Zhejiang Univ Sci B. 2024. PMID: 39420523 Free PMC article. Review.
Cited by
-
Accumulation Kinetics and Biological Action of Doxorubicin in Rabbit Intervertebral Discs.Int J Mol Sci. 2025 Jul 30;26(15):7386. doi: 10.3390/ijms26157386. Int J Mol Sci. 2025. PMID: 40806515 Free PMC article.
-
Screening of a Plant Extract Library from the Greek Flora for Biological Activities Related to Anti-Aging Applications.Antioxidants (Basel). 2025 Jul 4;14(7):824. doi: 10.3390/antiox14070824. Antioxidants (Basel). 2025. PMID: 40722928 Free PMC article.
References
-
- Ananthaswamy, H. N. , & Pierceall, W. E. (1990). Molecular mechanisms of ultraviolet radiation carcinogenesis. Photochemistry and Photobiology, 52(6), 1119–1136. - PubMed
-
- Armatas, A. A. , Pratsinis, H. , Mavrogonatou, E. , Angelopoulou, M. T. , Kouroumalis, A. , Karamanos, N. K. , & Kletsas, D. (2014). The differential proliferative response of fetal and adult human skin fibroblasts to TGF‐β is retained when cultured in the presence of fibronectin or collagen. Biochimica et Biophysica Acta, 1840(8), 2635–2642. - PubMed
-
- Bailey, A. D. , Gray, L. T. , Pavelitz, T. , Newman, J. C. , Horibata, K. , Tanaka, K. , & Weiner, A. M. (2012). The conserved Cockayne syndrome B‐piggyBac fusion protein (CSB‐PGBD3) affects DNA repair and induces both interferon‐like and innate antiviral responses in CSB‐null cells. DNA Repair (Amst), 11(5), 488–501. - PMC - PubMed
-
- Balajee, A. S. , Proietti De Santis, L. , Brosh, R. M., Jr. , Selzer, R. , & Bohr, V. A. (2000). Role of the ATPase domain of the Cockayne syndrome group B protein in UV induced apoptosis. Oncogene, 19(4), 477–489. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

