Sex-specific mechanisms of cerebral microvascular BKCa dysfunction in a mouse model of Alzheimer's disease
- PMID: 39698895
- PMCID: PMC11848394
- DOI: 10.1002/alz.14438
Sex-specific mechanisms of cerebral microvascular BKCa dysfunction in a mouse model of Alzheimer's disease
Abstract
Introduction: Cerebrovascular dysfunction occurs in Alzheimer's disease (AD), impairing hemodynamic regulation. Large conductance Ca2+-activated K+ channels (BKCa) regulate cerebrovascular reactivity and are impaired in AD. BKCa activity depends on intracellular Ca2+ (Ca2+ sparks) and nitro-oxidative post-translational modifications. However, whether these mechanisms underlie BKCa impairment in AD remains unknown.
Methods: Cerebral arteries from 5x-FAD and wild-type (WT) littermates were used for molecular biology, electrophysiology, ex vivo, and in vivo experiments.
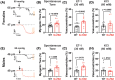
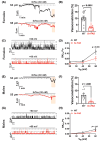
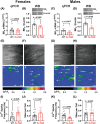
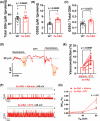
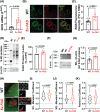
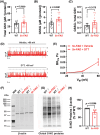
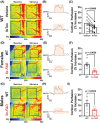
Results: Arterial BKCa activity is reduced in 5x-FAD via sex-dependent mechanisms: in males, there is lower BKα subunit expression and less Ca2+ sparks. In females, we observed reversible nitro-oxidative modification of BKCa. Further, BKCa is involved in hemodynamic regulation in WT mice, and its dysfunction is associated with vascular deficits in 5x-FAD.
Discussion: Our data highlight the central role played by BKCa in cerebral hemodynamic regulation and that molecular mechanisms of its impairment diverge based on sex in 5x-FAD.
Highlights: Cerebral microvascular BKCa dysfunction occurs in both female and male 5x-FAD. Reduction in BKα subunit protein and Ca2+ sparks drive the dysfunction in males. Nitro-oxidative stress is present in females, but not males, 5x-FAD. Reversible nitro-oxidation of BKα underlies BKCa dysfunction in female 5x-FAD.
Keywords: 5x‐FAD; BKCa channels; Ca+2 sparks; S‐nitrosylation; cerebral functional hyperemia; cerebral pial arteries; myogenic tone; post‐translational modifications.
© 2024 The Author(s). Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
The authors declare no conflicts of interest. Author disclosures are available in the supporting information.
Figures

Update of
-
Sex-specific mechanisms of cerebral microvascular BK Ca dysfunction in a mouse model of Alzheimer's disease.bioRxiv [Preprint]. 2024 May 9:2023.06.06.543962. doi: 10.1101/2023.06.06.543962. bioRxiv. 2024. Update in: Alzheimers Dement. 2025 Feb;21(2):e14438. doi: 10.1002/alz.14438. PMID: 37333104 Free PMC article. Updated. Preprint.
References
-
- 2023 Alzheimer's disease facts and figures. Alzheimers Dement. 2023;19(4):1598‐1695. - PubMed
-
- Jellinger KA. Alzheimer disease and cerebrovascular pathology: an update. J Neural Transm (Vienna). 2002;109(5‐6):813‐836. - PubMed
-
- Mehndiratta P, Manjila S, Ostergard T, et al. Cerebral amyloid angiopathy‐associated intracerebral hemorrhage: pathology and management. Neurosurg Focus. 2012;32(4):E7. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous

