Platelets as key cells in endometriosis patients: Insights from small extracellular vesicles in peritoneal fluid and endometriotic lesions analysis
- PMID: 39698929
- PMCID: PMC11656511
- DOI: 10.1096/fj.202402499R
Platelets as key cells in endometriosis patients: Insights from small extracellular vesicles in peritoneal fluid and endometriotic lesions analysis
Abstract
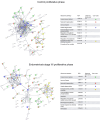
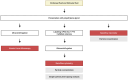
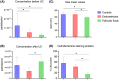
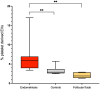
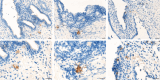
Endometriosis is a chronic inflammatory condition characterized by the presence of endometrium-like tissue outside the uterus, primarily affecting pelvic organs and tissues. In this study, we explored platelet activation in endometriosis. We utilized the STRING database to analyze the functional interactions among proteins previously identified in small extracellular vesicles (EVs) isolated from the peritoneal fluid of endometriosis patients and controls. The bioinformatic analysis indicated enriched signaling pathways related to platelet activation, hemostasis, and neutrophil degranulation. Double immunohistochemistry analysis for CD61 and MPO revealed a significant presence of neutrophils and platelets in close contact infiltrating endometriotic lesions, suggesting potential cell-cell interactions. Subsequently, we isolated small EVs from the peritoneal fluid of women diagnosed with endometriosis and from women without endometriosis who underwent surgery for non-inflammatory benign diseases. We performed single-particle phenotyping analysis based on platelet biomarkers GPIIb/IIIa and PF4 using nanoflow cytometry, as well as single-particle morphological and nanomechanical characterization through atomic force microscopy. The study demonstrated that patients with endometriosis had a notably higher proportion of particles testing positive for platelet biomarkers compared to the total number of EVs. This finding implies a potential role for platelets in the pathogenesis of endometriosis. Further research is necessary to delve into the mechanisms underlying this phenomenon and its implications for disease progression.
Keywords: GPIIb/IIIa; PF4; endometriosis; extracellular vesicles; follicular fluid; neutrophil; peritoneal fluid; platelet.
© 2024 The Author(s). The FASEB Journal published by Wiley Periodicals LLC on behalf of Federation of American Societies for Experimental Biology.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

