Mycobiome analyses of critically ill COVID-19 patients
- PMID: 39699254
- PMCID: PMC11792475
- DOI: 10.1128/spectrum.04110-23
Mycobiome analyses of critically ill COVID-19 patients
Abstract
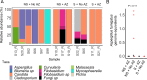
Coronavirus disease 2019 (COVID-19)-associated pulmonary aspergillosis (CAPA) is a life-threatening complication in patients with severe COVID-19. Previously, acute respiratory distress syndrome in patients with COVID-19 has been associated with lung fungal dysbiosis, evidenced by reduced microbial diversity and Candida colonization. Increased fungal burden in the lungs of critically ill COVID-19 patients is linked to prolonged mechanical ventilation and increased mortality. However, specific mycobiome signatures associated with severe COVID-19 in the context of survival and antifungal drug prophylaxis have not yet been determined, and such knowledge could have an important impact on treatment. To understand the composition of the respiratory mycobiome in critically ill COVID-19 patients with and without CAPA and the impact of antifungal use in patient outcome, we performed a multinational study of 39 COVID-19 patients in intensive care units (ICUs). Respiratory mycobiome was profiled using internal transcribed spacer 1 sequencing, and Aspergillus fumigatus burden was further validated using quantitative PCR. Fungal communities were investigated using alpha diversity, beta diversity, taxa predominance, and taxa abundances. Respiratory mycobiomes of COVID-19 patients were dominated by Candida and Aspergillus. There was no significant association with corticosteroid use or CAPA diagnosis and respiratory fungal communities. Increased A. fumigatus burden was associated with mortality and, the use of azoles at ICU admission was linked with an absence of A. fumigatus. Our findings suggest that mold-active antifungal treatment at ICU admission may be linked with reduced A. fumigatus-associated mortality in severe COVID-19. However, further studies are warranted on this topic.IMPORTANCEInvasive fungal infections are a serious complication affecting up to a third of patients with severe COVID-19. Nevertheless, our understanding of the fungal communities in the lungs during critically ill COVID-19 remains limited. Evidence suggests a higher fungal burden is associated with prolonged ventilation and higher mortality, although the particular organisms responsible for this link are unclear. Antifungal prophylaxis may be beneficial for reducing the burden of fungal co-infections in COVID-19 intensive care. However, the composition of the fungal microbiome in severe COVID-19 in relation to prophylactic antifungals, as well as how this is associated with survival outcomes, is yet to be studied. Our study provides insights into the lung fungal microbiome in severe COVID-19 and has found antifungal treatment to be associated with lower Aspergillus fumigatus burden and that higher levels of this pathogen are associated with mortality. Therefore, our study suggests mold-active antifungal prophylaxis may be beneficial in severe COVID-19.
Keywords: Aspergillus; CAPA; COVID-19; mycobiome.
Conflict of interest statement
M.H. received research funding from Gilead, Astellas, MSD, Euroimmune, IMMY, Scynexis, Pulmocide, F2G, and Pfizer outside the submitted work. M.J.B. is a former employee and has previously received research funding from F2G outside the submitted work. In the past 5 years, S.G. has received speaker fees from Gilead Sciences and research grant support from Pfizer outside of the submitted work. Outside the submitted work, D.R.G. reports investigator-initiated grants from Pfizer, Shionogi, and Gilead Italia and speaker and/or advisor fees from Pfizer and Tillotts Pharma. Outside the submitted work, M.B. reports research grants and/or personal fees for advisor/consultant and/or speaker/chairman from Bayer, BioMérieux, Cidara, Cipla, Gilead, Menarini, MSD, Pfizer, and Shionogi. In the past 5 years, J.-P.G. has received speaker fees from Gilead Sciences, MundiPharma, Pfizer, and Shionogi outside of the submitted work. In the past 5 years, H.G. has received speaker fees from Gilead Sciences. J.P. has received speakers' fees from Gilead Sciences, Pfizer, Swedish Orphan Biovitrum, and Associated of Cape Cod outside of the submitted work; has served at advisor boards at Gilead Sciences and Pfizer; and holds stocks of NovoNordisk and AbbVie Inc. All remaining authors declare no competing interests.
Figures



References
-
- World Health Organisation . 2024. WHO coronavirus dashboard. Available from: https://covid19.who.int/
-
- Osuchowski MF, Winkler MS, Skirecki T, Cajander S, Shankar-Hari M, Lachmann G, Monneret G, Venet F, Bauer M, Brunkhorst FM, et al. 2021. The COVID-19 puzzle: deciphering pathophysiology and phenotypes of a new disease entity. Lancet Respir Med 9:622–642. doi: 10.1016/S2213-2600(21)00218-6 - DOI - PMC - PubMed
-
- Dongelmans DA, Termorshuizen F, Brinkman S, Bakhshi-Raiez F, Arbous MS, de Lange DW, van Bussel BCT, de Keizer NF, Dutch COVID-19 Research Consortium . 2022. Characteristics and outcome of COVID-19 patients admitted to the ICU: a nationwide cohort study on the comparison between the first and the consecutive upsurges of the second wave of the COVID-19 pandemic in the Netherlands. Ann Intensive Care 12:5. doi: 10.1186/s13613-021-00978-3 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

