Neohesperidin Dihydrochalcone Alleviates Lipopolysaccharide-Induced Vascular Endothelium Dysfunction by Regulating Antioxidant Capacity
- PMID: 39699295
- PMCID: PMC11656606
- DOI: 10.1002/iid3.70107
Neohesperidin Dihydrochalcone Alleviates Lipopolysaccharide-Induced Vascular Endothelium Dysfunction by Regulating Antioxidant Capacity
Abstract
Background: Endothelial dysfunction is one of the important mechanisms of organ and tissue damage in sepsis. In this study, we evaluated the effects of neohesperidin dihydrochalone (NHDC) on lipopolysaccharide (LPS)-induced vascular dysfunction and explored the potential mechanisms.
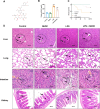
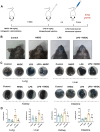
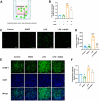
Methods: In vivo, we assessed vascular leakage in mice by injecting Evans blue dye. In vitro, cell counting kit-8 (CCK-8) assay and flow cytometry were used to assess the activity of HUVEC and apoptosis. The effect of LPS on HUVEC barrier was assessed using FITC-extend membrane assay. The adhesion ability of HUVEC was tested by THP-1 cell adhesion assay. The antioxidant capacity of cells was measured by detecting the level of mitochondrial membrane potential, ROS, and content of CAT, SOD, GSH, and MDA within the cells. Furthermore, the release of endothelial IL-1β, IL-6, and TNF-α were detected by ELISA, and the expression level of TAK1, ERK1/2, and NFκB were detected by western blot.
Results: Treatment with NHDC effectively alleviated LPS-induced endothelial permeability and organ damage by reducing reactive oxygen species production and enhancing the antioxidant response. Further investigation suggested that NHDC may exert its protective effects by inhibiting the release of IL-1β, IL-6, and TNF-α, and by decreasing the phosphorylation of key inflammatory signaling molecules, including transforming growth factor-β-activated kinase 1 (TAK1), extracellular signal-regulated kinases 1/2 (ERK1/2), and nuclear factor kappa B (NFκB).
Conclusions: Our study indicate that pretreatment with NHDC may provide protection against LPS-induced vascular dysfunction by reducing oxidative stress and activation of inflammatory signaling pathways.
Keywords: endothelial dysfunction; inflammation; neohesperidin dihydrochalcone; oxidative stress; sepsis.
© 2024 The Author(s). Immunity, Inflammation and Disease published by John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures




References
-
- Lelubre C. and Vincent J. L., “Mechanisms and Treatment of Organ Failure in Sepsis,” Nature Reviews Nephrology 14, no. 7 (2018): 417–427. - PubMed
-
- Taghavi S., Abdullah S., Shaheen F., et al., “Exosomes and Microvesicles From Adipose‐Derived Mesenchymal Stem Cells Protects the Endothelial Glycocalyx From Lps Injury,” Shock 60, no. 1 (2023): 56–63. - PubMed
-
- Khakpour S., Wilhelmsen K., and Hellman J., “Vascular Endothelial Cell Toll‐Like Receptor Pathways in Sepsis,” Innate Immunity 21, no. 8 (2015): 827–846. - PubMed
-
- Frydman A., Weisshaus O., Huhman D. V., et al., “Metabolic Engineering of Plant Cells for Biotransformation of Hesperedin Into Neohesperidin, a Substrate for Production of the Low‐Calorie Sweetener and Flavor Enhancer NHDC,” Journal of Agricultural and Food Chemistry 53, no. 25 (2005): 9708–9712. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

