Lysosomal-Mitochondrial Interaction Promotes Tumor Growth in Squamous Cell Carcinoma of the Head and Neck
- PMID: 39699311
- PMCID: PMC11961326
- DOI: 10.1158/1541-7786.MCR-24-0337
Lysosomal-Mitochondrial Interaction Promotes Tumor Growth in Squamous Cell Carcinoma of the Head and Neck
Abstract
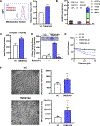
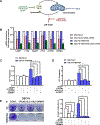
Communication between intracellular organelles including lysosomes and mitochondria has recently been shown to regulate cellular proliferation and fitness. The way lysosomes and mitochondria communicate with each other [lysosomal-mitochondrial interaction (LMI)] is emerging as a major determinant of tumor proliferation and growth. About 30% of squamous carcinomas [including squamous cell carcinoma of the head and neck (SCCHN)] overexpress transmembrane member 16A (TMEM16A), a calcium-activated chloride channel, which promotes cellular growth and negatively correlates with patient survival. We have recently shown that TMEM16A drives lysosomal biogenesis; however, its impact on mitochondrial function has not been explored. In this study, we show that in the context of high-TMEM16A SCCHN, (i) patients display increased mitochondrial content, specifically complex I; (ii) in vitro and in vivo models uniquely depend on mitochondrial complex I activity for growth and survival; (iii) NRF2 signaling is a critical linchpin that drives mitochondrial function, and (iv) mitochondrial complex I and lysosomal function are codependent for proliferation. Taken together, our data demonstrate that coordinated lysosomal and mitochondrial activity and biogenesis via LMI drive tumor proliferation and facilitate a functional interaction between lysosomal and mitochondrial networks. Therefore, inhibition of LMI instauration may serve as a therapeutic strategy for patients with SCCHN. Implications: Intervention of LMI may serve as a therapeutic approach for patients with high TMEM16A-expressing SCCHN.
©2024 American Association for Cancer Research.
Conflict of interest statement
Competing Interest
The authors declare no conflict of interest.
Figures



Update of
-
Lysosomal mitochondrial interaction promotes tumor growth in squamous cell carcinoma of the head and neck.bioRxiv [Preprint]. 2023 Jun 26:2023.06.25.546311. doi: 10.1101/2023.06.25.546311. bioRxiv. 2023. Update in: Mol Cancer Res. 2025 Apr 01;23(4):339-349. doi: 10.1158/1541-7786.MCR-24-0337. PMID: 37425842 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

