Five-Year Outcomes of Lenadogene Nolparvovec Gene Therapy in Leber Hereditary Optic Neuropathy
- PMID: 39699886
- PMCID: PMC11843360
- DOI: 10.1001/jamaophthalmol.2024.5375
Five-Year Outcomes of Lenadogene Nolparvovec Gene Therapy in Leber Hereditary Optic Neuropathy
Abstract
Importance: Limited studies have assessed the long-term benefit/risk of gene therapy for Leber hereditary optic neuropathy (LHON).
Objective: To determine the safety and efficacy of lenadogene nolparvovec in patients with LHON due to the MT-ND4 gene variant for up to 5 years after administration.
Design, setting, and participants: The RESCUE and REVERSE Long-Term Follow-up Study (RESTORE), conducted from 2018 to 2022, is the 5-year follow-up study of the 2 phase 3 clinical studies RESCUE (Efficacy Study of Lenadogene Nolparvovec for the Treatment of Vision Loss Up to 6 Months From Onset in LHON Due to the MT-ND4 Mutation) and REVERSE (Efficacy Study of Lenadogene Nolparvovec for the Treatment of Vision Loss From 7 Months to 1 Year From Onset in LHON Due to the MT-ND4 Mutation). At the end of each study, ie, 2 years after gene therapy administration, patients were offered enrollment in the RESTORE trial, a multinational, multicenter, prospective study, for an additional 3 years of follow-up. Patients with LHON due to the MT-ND4 gene variant received lenadogene nolparvovec in 1 eye and a sham injection in the other eye.
Intervention: Lenadogene nolparvovec was administered as a single intravitreal injection in the RESCUE/REVERSE studies.
Main outcomes and measures: Measures included best-corrected visual acuity (BCVA), quality of life using the National Eye Institute visual functioning questionnaire 25 (NEI VFQ-25), and adverse events.
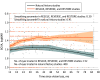
Results: Among the 76 patients who received gene therapy in the RESCUE (n = 39) and REVERSE (n = 37) studies, 72 (94.7%) completed these studies; 62 patients (81.6%) participated in the RESTORE trial, and 55 patients (72.4%) completed the 5-year follow-up. Participants were mostly male (49 [79.0%]) with a mean (SD) age of 35.9 (15.3) years at treatment. At baseline, the mean (SD) BCVA was 1.5 (0.5) logMAR (20/600 Snellen) in eyes to be treated with lenadogene nolparvovec and 1.4 (0.5) logMAR (20/500) in sham eyes. At the end of the RESCUE/REVERSE trials, ie, 2 years after treatment, eyes treated with lenadogene nolparvovec and eyes treated with sham reached a mean BCVA value of 1.4 (0.6) logMAR (20/500). The mean (SD) change from baseline to year 2 was -0.05 (0.6) logMAR (+1 line) and 0.01 (0.6) logMAR (-0 line) in gene therapy-treated and sham eyes, respectively (difference, -0.03; 95% CI, -0.16 to 0.09; P = .60). Five years after treatment, the bilateral improvement from nadir was similar to that observed at 2 years, with a mean (SD) change in BCVA of -0.4 (0.5) logMAR (more than +4 lines) for eyes treated with lenadogene nolparvovec and -0.4 (0.4) logMAR (+4 lines) for eyes treated with sham (difference, -0.05; 95% CI, -0.15 to 0.04; P = .27). An improvement of at least -0.3 logMAR (+3 lines) from the nadir in at least 1 eye was observed in 66.1% of participants (41 of 62). Between 2 and 5 years, intraocular inflammation was noted in 4 participants with 8 events in eyes treated with lenadogene nolparvovec and 1 event in an eye treated with sham.
Conclusions and relevance: In this analysis of the RESTORE trial, follow-up of patients with LHON due to the MT-ND4 gene variant unilaterally treated with lenadogene nolparvovec demonstrated a sustained bilateral improvement in BCVA and a good safety profile up to 5 years after treatment. This evidence of persistent benefit over time is promising for the use of gene therapy in these patients.
Trial registration: ClinicalTrials.gov Identifier: NCT03406104.
Conflict of interest statement
Figures

Comment on
-
Single-Eye Gene Therapy for Leber Hereditary Optic Neuropathy.JAMA Ophthalmol. 2025 Feb 1;143(2):108-109. doi: 10.1001/jamaophthalmol.2024.5618. JAMA Ophthalmol. 2025. PMID: 39699916 No abstract available.
References
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

