Dysregulated alveolar epithelial cell progenitor function and identity in Hermansky-Pudlak syndrome
- PMID: 39699958
- PMCID: PMC11948584
- DOI: 10.1172/jci.insight.183483
Dysregulated alveolar epithelial cell progenitor function and identity in Hermansky-Pudlak syndrome
Abstract
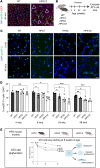
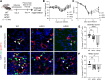
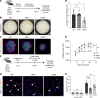
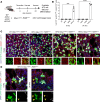
Hermansky-Pudlak syndrome (HPS) is a genetic disorder of endosomal protein trafficking associated with pulmonary fibrosis in specific subtypes, including HPS-1 and HPS-2. Single-mutant HPS1 and HPS2 mice display increased fibrotic sensitivity while double-mutant HPS1/2 mice exhibit spontaneous fibrosis with aging, which has been attributed to HPS mutations in alveolar epithelial type II (AT2) cells. We utilized HPS mouse models and human lung tissue to investigate mechanisms of AT2 cell dysfunction driving fibrotic remodeling in HPS. Starting at 8 weeks of age, HPS mice exhibited progressive loss of AT2 cell numbers. HPS AT2 cell function was impaired ex vivo and in vivo. Incorporating AT2 cell lineage tracing in HPS mice, we observed aberrant differentiation with increased AT2-derived alveolar epithelial type I cells. Transcriptomic analysis of HPS AT2 cells revealed elevated expression of genes associated with aberrant differentiation and p53 activation. Lineage-tracing and organoid-modeling studies demonstrated that HPS AT2 cells were primed to persist in a Keratin-8-positive reprogrammed transitional state, mediated by p53 activity. Intrinsic AT2 progenitor cell dysfunction and p53 pathway dysregulation are mechanisms of disease in HPS-related pulmonary fibrosis, with the potential for early targeted intervention before the onset of fibrotic lung disease.
Keywords: Cell biology; Fibrosis; Genetic diseases; Lysosomes; Pulmonology.
Conflict of interest statement
Figures

Update of
-
Dysregulated alveolar epithelial cell progenitor function and identity in Hermansky-Pudlak syndrome.bioRxiv [Preprint]. 2024 Dec 14:2023.06.17.545390. doi: 10.1101/2023.06.17.545390. bioRxiv. 2024. Update in: JCI Insight. 2024 Dec 19;10(3):e183483. doi: 10.1172/jci.insight.183483. PMID: 38496421 Free PMC article. Updated. Preprint.
References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

