Growth hormone receptor in VGLUT2 or Sim1 cells regulates glycemia and insulin sensitivity
- PMID: 39700135
- PMCID: PMC11670237
- DOI: 10.1073/pnas.2407225121
Growth hormone receptor in VGLUT2 or Sim1 cells regulates glycemia and insulin sensitivity
Abstract
Growth hormone (GH) has several metabolic effects, including a profound impact on glucose homeostasis. For example, GH oversecretion induces insulin resistance and increases the risk of developing diabetes mellitus. Here, we show that GH receptor (GHR) ablation in vesicular glutamate transporter 2 (VGLUT2)-expressing cells, which comprise a subgroup of glutamatergic neurons, led to a slight decrease in lean body mass without inducing changes in body adiposity. VGLUT2∆GHR mice exhibited reduced glycemia and improved glucose tolerance and insulin sensitivity. Among different glutamatergic neuronal populations, we found that GHR inactivation in Sim1-expressing cells recapitulated the phenotype observed in VGLUT2∆GHR mice. Furthermore, Sim1∆GHR mice exhibited reduced endogenous glucose production and improved hepatic insulin sensitivity without alterations in whole-body or muscle glucose uptake. Sim1∆GHR mice were protected against acute but not chronic diabetogenic effects of exogenous GH administration. Pharmacological activation of ATP-sensitive potassium channels in the brain normalized blood glucose levels in Sim1∆GHR mice. In conclusion, the absence of GHR signaling in VGLUT2/Sim1-expressing cells causes a persistent reduction in glycemia and improves hepatic insulin sensitivity. Central glucose-sensing mechanisms are likely involved in the reduced glycemia exhibited by Sim1∆GHR mice. The current findings uncover a mechanism involved in the effects of GHR signaling in regulating glucose homeostasis.
Keywords: GH; diabetes mellitus; glucose metabolism; insulin.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures
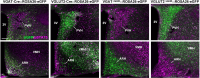


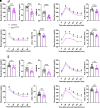


References
-
- Houssay B. A., Anderson E., Diabetogenic action of purified anterior pituitary hormones. Endocrinology 45, 627–629 (1949). - PubMed
-
- Houssay B. A., Biasotti A., The hypophysis, carbohydrate metabolism and diabetes. Endocrinology 15, 511–523 (1931).
-
- Houssay B. A., Penhos J. C., Diabetogenic action of pituitary hormones on adrenalectomized hypophysectomized dogs. Endocrinology 59, 637–641 (1956). - PubMed
-
- Houssay B. A., Rodriguez R. R., Diabetogenic action of different preparations of growth hormone. Endocrinology 53, 114–116 (1953). - PubMed
-
- Zierler K. L., Rabinowitz D., Roles of insulin and growth hormone, based on studies of forearm metabolism in man. Medicine (Baltimore) 42, 385–402 (1963). - PubMed
MeSH terms
Substances
Grants and funding
- 2018/04956-5/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 306024/2023-3/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
- Finance Code 001/Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES)
- R01 AG059779/AG/NIA NIH HHS/United States
- 2021/08792-0/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2020/10102-9/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 313752/2023-0/Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq)
- 2020/01318-8/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- 2022/11262-5/Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP)
- R01AG059779/HHS | NIH (NIH)
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases

