Alveolar macrophages are early targets of mumps virus
- PMID: 39700136
- PMCID: PMC11670124
- DOI: 10.1073/pnas.2410954121
Alveolar macrophages are early targets of mumps virus
Abstract
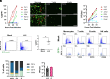
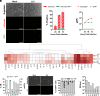
Formerly a common childhood pathogen, mumps virus (MuV) remains active worldwide, despite relatively high vaccine coverage. MuV is thought to infect the upper respiratory tract before disseminating to other organs; however, the early cellular targets of MuV in vivo are unknown. To address this, we generated a green fluorescent protein (GFP)-tagged vaccine strain (JL5) of MuV to infect leukocytic cell lines and found that replication was greatest in monocytes. Infection of peripheral blood mononuclear cells (PBMCs) also showed that both JL5 and a circulating strain of MuV (Iowa 2006; genotype G), preferentially infected monocytes. Further, monocyte-derived macrophages showed high susceptibility to MuV, with genotype G infecting macrophages to a much greater extent. While mice are generally resistant to MuV infection, we inoculated immunocompetent Rosa26-tdTomato mice intranasally with a GFP and Cre recombinase tagged MuV to determine whether monocytes/macrophages are important targets in vivo. We observed a small population of tdTomato+ cells within the lungs, which included epithelial cells; however, the vast majority were alveolar macrophages (AMs). To validate these findings, we infected murine AMs isolated from Rosa26-tdTomato mice with the GFP and Cre recombinase tagged MuV and found that while MuV could enter AMs, as determined by tdTomato positivity, only a small percentage of these expressed GFP, suggesting that inhibition in murine cells occurs postentry. To translate these findings, we infected cells from human bronchoalveolar lavage fluid with MuV and found that most infected cells were AMs. These findings highlight the high susceptibility of AMs and provide a basis for early MuV pathogenesis and subsequent dissemination.
Keywords: entry; lung; mouse; myeloid; pathogenesis.
Conflict of interest statement
Competing interests statement:The authors declare no competing interest.
Figures


References
MeSH terms
Substances
Grants and funding
- PR192188/U.S. Department of Defense (DOD)
- R01AI188431/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- U19AI171403/HHS | NIH | National Institute of Allergy and Infectious Diseases (NIAID)
- T32 GM146636/GM/NIGMS NIH HHS/United States
- U19 AI171403/AI/NIAID NIH HHS/United States
LinkOut - more resources
Full Text Sources
Medical

