Affinity maturation of antibody responses is mediated by differential plasma cell proliferation
- PMID: 39700316
- PMCID: PMC11938350
- DOI: 10.1126/science.adr6896
Affinity maturation of antibody responses is mediated by differential plasma cell proliferation
Abstract
Increased antibody affinity over time after vaccination, known as affinity maturation, is a prototypical feature of immune responses. Recent studies have shown that a diverse collection of B cells, producing antibodies with a wide spectrum of different affinities, is selected into the plasma cell (PC) pathway. How affinity-permissive selection enables PC affinity maturation remains unknown. We found that PC precursors (prePCs) expressing high-affinity antibodies received higher levels of T follicular helper cell (TFH cell)-derived help and divided at higher rates compared with their lower-affinity counterparts once they left the germinal center. Our findings indicate that differential cell division by selected prePCs accounts for how diverse precursors develop into a PC compartment that mediates serological affinity maturation.
Conflict of interest statement
Competing interests
MCN is on the scientific advisory board of Celldex Therapeutics. All other authors declare that they have no competing interests.
Figures


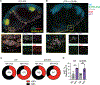

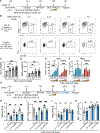
Update of
-
Affinity maturation of antibody responses is mediated by differential plasma cell proliferation.bioRxiv [Preprint]. 2024 Nov 29:2024.11.26.625430. doi: 10.1101/2024.11.26.625430. bioRxiv. 2024. Update in: Science. 2025 Jan 24;387(6732):413-420. doi: 10.1126/science.adr6896. PMID: 39651284 Free PMC article. Updated. Preprint.
References
-
- Chan TD et al. , Antigen affinity controls rapid T-dependent antibody production by driving the expansion rather than the differentiation or extrafollicular migration of early plasmablasts. J Immunol 183, 3139–3149 (2009). - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

