Radiographic and visual response to the type II RAF inhibitor tovorafenib in children with relapsed/refractory optic pathway glioma in the FIREFLY-1 trial
- PMID: 39700439
- PMCID: PMC12187376
- DOI: 10.1093/neuonc/noae274
Radiographic and visual response to the type II RAF inhibitor tovorafenib in children with relapsed/refractory optic pathway glioma in the FIREFLY-1 trial
Abstract
Background: Due to their anatomical locations, optic pathway gliomas (OPGs) can rarely be cured by resection. Given the importance of preserving visual function, we analyzed radiological and visual acuity (VA) outcomes for the type II RAF inhibitor tovorafenib in the OPG subgroup of the phase 2 FIREFLY-1 trial.
Methods: FIREFLY-1 investigated the efficacy (arm 1, n = 77), safety, and tolerability (arms 1/2) of tovorafenib (420 mg/m2 once weekly; 600 mg maximum) in patients with BRAF-altered relapsed/refractory pediatric low-grade glioma (pLGG). In this post hoc analysis, anti-tumor activity and VA were analyzed in arm 1 patients with OPG. Anti-tumor activity was independently assessed per Response Assessment in Neuro-Oncology high-grade glioma (RANO-HGG), Response Assessment in Pediatric Neuro-Oncology-LGG (RAPNO), and RANO-LGG criteria. The data cutoff was June 5, 2023.
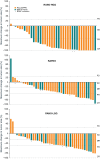
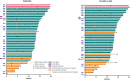
Results: Forty-two of 77 patients had OPGs; 35 of 42 had ≥2 VA assessments. The overall response rate in the OPG subgroup according to RANO-HGG, RAPNO, and RANO-LGG criteria were 64%, 50%, and 55%, with clinical benefit rates of 95%, 88%, and 90%, respectively. VA per patient was preserved for 80% of patients; 31% demonstrated improved VA; VA per eye was preserved in 87%, with 27% improving. The safety profile in the arm 1 OPG subgroup was similar to the overall FIREFLY-1 safety analysis set.
Conclusions: Tovorafenib demonstrated anti-tumor activity in relapsed/refractory BRAF-altered OPG across radiological assessment criteria and was generally well tolerated. Importantly, vision remained stable or improved in most patients.
Keywords: BRAF; FIREFLY-1; optic pathway glioma; tovorafenib; visual acuity.
© The Author(s) 2024. Published by Oxford University Press on behalf of the Society for Neuro-Oncology.
Conflict of interest statement
K.N. reports scientific writing support paid for by Day One, contracts between his employer and Amgen, Astra Zeneca, Daiichi-Sankyo, Lilly, Merck/MSD, Novartis, Pfizer, Roche, Turning Point, Y-mAbs in relation to conducting clinical trials where he is the national principal investigator, personal payment from Bayer, support for meeting attendance from Day One, personal payments in relation to service on the data monitoring committee of a clinical trial sponsored by Lilly, and non-compensated roles as a member of the Executive Committee of the Innovative Therapies for Children with Cancer (ITCC), chair of the Novel Therapy working group of the Nordic Society for Pediatric Hematology & Oncology (NOPHO), and chair of the multidisciplinary working group on central nervous system tumors of children in Denmark. L.B.K. reports contracted institutional research from Day One Biopharmaceuticals, support from Day One Biopharmaceuticals in relation to providing resources to complete the post hoc analysis, manage the manuscript development, and fund the medical writing, contracted institutional research from Novartis, Regeneron Pharmaceuticals, SpringWorks Therapeutics, Bristol Myers Squibb, SonALAsense, Recursion, and Chimerix, consulting fees from Blueprint Medicines as DSMB Chair, and Onconova Therapeutics stock ownership. S.E.S.L. reports support in relation to this manuscript (preparation, writing, journal charges) from Day One, and a clinical trial agreement with Day One supporting the conduct of the trial at the site, including provision of drug and research costs (no personal payment as an investigator). O.W. reports research grants to his institution from Day One Biopharmaceuticals and Biomede Valley Discovery, personal consulting fees from Novartis and Roche, a leadership role with SIOPE BTG in relation to the LOGGIC consortium, and a co-leadership role of the pLGG study group of the GPOH. D.S.Z. reports personal consulting fees from Novartis, FivePhusion, Amgen, and Accendatech, and participation in data safety monitoring or advisory boards for Day One, Alexion, and Norgine. P.H.D. reports a grant/contract from IPSEN, personal consulting fees from IPSEN and consulting fees paid to his institution from ALEXION and Bayer, and participation on a data safety monitoring board/advisory board for Biomede. A.T.F. reports participation on an advisory board for Day One Biopharmaceuticals. C.K. reports grants from Cannonball Kids’ Cancer Foundation, Kortney Rose Foundation, and Bristol-Myers Squibb Co, contracts related to clinical trials from Curis Inc, Regeneron Pharmaceuticals, Day One Biotherapeutics, Midatech, Ipsen, Chimerix, Kazia, and Bristol-Myers Squibb Co, and participation on scientific advisory boards for Cannonball Kids’ Cancer Foundation, Raymond A. Wood Foundation, and Children’s Brain Tumor Network. D.S. reports payment for participation on an advisory board from Alexion. G.M. reports that Day One Biopharmaceuticals funded the FIREFLY-1 trial and provided resources to complete the post hoc analysis, manage the manuscript development, and fund the medical writing. J.R.H. reports receiving personal consulting fees from Bayer Pharmaceuticals and Alexion Pharmaceuticals, institutional honoraria for scientific advisory boards from Servier International, and support for attending meetings and/or travel from Alexion Pharmaceuticals. N.G.G. has received payment/honoraria from Bayer and is the Board Chair of ANZCHOG (Australian and New Zealand Children’s Haematology and Oncology Group). T.H. reports that Day One Biopharmaceuticals funded the FIREFLY-1 trial and provided resources to complete the post hoc analysis, manage the manuscript development, and fund the medical writing. J.Q., D.D.C., S.G.R., and P.M. are employees of Day One Biopharmaceuticals and have received Day One Biopharmaceuticals stock/stock options. D.H. reports clinical trial funding for selumetinib (institutional) from AstraZeneca/Alexion, personal consulting fees from AstraZeneca/Alexion, Novartis, and Day One Biotherapeutics, personal payment/honoraria from Alexion, support for attending meetings and/or travel from AstraZeneca/Alexion and Novartis, and participation on a data safety monitoring board or advisory board from AstraZeneca, Novartis, and Day One Biotherapeutics. D.B.L., E.dV.K., S.P., P.A.B., N.S.W., N.J., S.B., D.A.K.Q., J.W.H., M.Y.O., S.N.C., have declared no conflict of interest.
Figures

References
-
- Gnekow AK, Kandels D, Tilburg CV, et al. SIOP-E-BTG and GPOH guidelines for diagnosis and treatment of children and adolescents with low grade glioma. Klin Padiatr. 2019;231(3):107–135. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

