Long-term efficacy and safety of danicopan as add-on therapy to ravulizumab or eculizumab in PNH with significant EVH
- PMID: 39700502
- PMCID: PMC11867134
- DOI: 10.1182/blood.2024026299
Long-term efficacy and safety of danicopan as add-on therapy to ravulizumab or eculizumab in PNH with significant EVH
Abstract
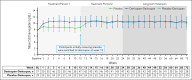
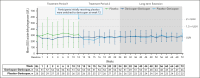
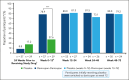
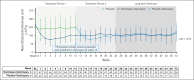
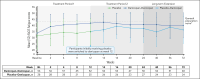
Complement C5 inhibitor treatment with ravulizumab or eculizumab for paroxysmal nocturnal hemoglobinuria (PNH) improves outcomes and survival. Some patients remain anemic due to clinically significant extravascular hemolysis (cs-EVH; hemoglobin [Hb] ≤9.5 g/dL and absolute reticulocyte count [ARC] ≥120 × 109/L). In the phase 3 ALPHA trial, participants received oral factor D inhibitor danicopan (150 mg 3 times daily) or placebo plus ravulizumab or eculizumab during the 12-week, double-blind treatment period 1 (TP1); those receiving placebo switched to danicopan during the subsequent 12-week, open-label TP2 and continued during the 2-year long-term extension (LTE). There were 86 participants randomized in the study, of whom 82 entered TP2, and 80 entered LTE. The primary end point was met, with Hb improvements from baseline at week 12 (least squares mean change, 2.8 g/dL) with danicopan. For participants switching from placebo to danicopan at week 12, improvements in mean Hb were observed at week 24. Similar trends were observed for the proportion of participants with ≥2 g/dL Hb increase, ARC, proportion of participants achieving transfusion avoidance, and Functional Assessment of Chronic Illness Therapy-Fatigue scale scores. Improvements were maintained up to week 72. No new safety signals were observed. The breakthrough hemolysis rate was 6 events per 100 patient-years. These long-term data demonstrate sustained efficacy and safety of danicopan plus ravulizumab/eculizumab for continued control of terminal complement activity, intravascular hemolysis, and cs-EVH in PNH. This trial was registered at www.clinicaltrials.gov as #NCT04469465.
© 2025 American Society of Hematology. Published by Elsevier Inc. Licensed under Creative Commons Attribution-NonCommercial-NoDerivatives 4.0 International (CC BY-NC-ND 4.0), permitting only noncommercial, nonderivative use with attribution. All other rights reserved.
Conflict of interest statement
Conflict-of-interest disclosure: A.K. has received honoraria from Alexion, AstraZeneca Rare Disease, Amgen, Agios, Celgene/Bristol Myers Squibb (BMS), Novartis, Pfizer, Roche, Samsung, and Sobi; is on the board of directors or is an advisory board member for Alexion, AstraZeneca Rare Disease, Amgen, BioCryst, Celgene/BMS, Novartis, Regeneron, and Roche; and has received consulting fees from Alexion, AstraZeneca Rare Disease, Celgene/BMS, Novo Nordisk, Janssen Pharmaceuticals, Pfizer, Roche, Samsung, Sobi, and Novartis. M.G. has received honoraria from Alexion, AstraZeneca Rare Disease, Sobi, and Pfizer; has served as an advisory board member for Alexion, AstraZeneca Rare Disease, Amgen, BioCryst, Novartis, Pfizer, and Sobi; has served as a consultant for BioCryst and Regeneron Pharmaceuticals; and has received educational grant support from Apellis. C. Piatek has served as a consultant to Alexion, AstraZeneca Rare Disease; has served on board or advisory committees for Annexon Biosciences, Apellis, Alexion, AstraZeneca Rare Disease, Rigel, Sanofi, and Sobi; has served on a speakers bureau for Sobi; and has received research funding from Argenx, Alexion, AstraZeneca Rare Disease, Celgene, Oscotec, Rigel, Sanofi, and Incyte. J.S. has received honoraria and consulting fees from Alexion, AstraZeneca Rare Disease, Apellis, BMS, CTI Bio, GlaxoSmithKline, Incyte, Novartis, and Sanofi. J.-i.N. has received grants from Alexion, AstraZeneca Rare Disease; is an advisory board member for Alexion, AstraZeneca Rare Disease, Chugai, and Roche; and has received honoraria from Alexion, AstraZeneca Rare Disease. C. Patriquin has received honoraria for participation in speaker bureaus and/or consulting from Alexion, AstraZeneca Rare Disease, Apellis, Amgen, BioCryst, Novartis, Roche, Sanofi, Sobi, and Takeda and has served as a clinical site investigator for Alexion, AstraZeneca Rare Disease and Apellis. H.S. has received travel support, honoraria, and research support (to institution) from Alexion, AstraZeneca Rare Disease, Novartis, and Sobi and honoraria (to University of Ulm) from Alexion, AstraZeneca Rare Disease, Apellis, Roche, Sanofi, and Sobi. W.B. has served as a consultant to Alexion, AstraZeneca Rare Disease, Novartis, Roche, Sanofi, and Sobi and received research funding from Alexion. J.P. has received honoraria from and is an advisory board member for Alexion, Amgen, Apellis, AstraZeneca, Blueprint Medicines, BMS, Boehringer Ingelheim, Gilead, Merck Sharp & Dohme, Novartis, Pfizer, Roche, Sanofi, and Sobi; has received travel support from Alexion, Blueprint Medicines, Boehringer Ingelheim, Novartis, Pfizer, Roche, and Sobi; has served in a leadership or fiduciary role for the Lichterzellen Foundation; and has received other support from Apellis, Blueprint Medicines, Novartis, and Roche. A.G. has received honoraria from Alexion, AstraZeneca Rare Disease, Novartis, Roche, and Sobi and is an advisory board member for Alexion, AstraZeneca Rare Disease, Novartis, Roche, and Sobi. Y.P., P.L., and G.F. are employees of Alexion, AstraZeneca Rare Disease. F.S.d.F. has received honoraria and research support (to Saint-Louis Hospital, Paris, France) from Alexion, AstraZeneca Rare Disease, Novartis, Samsung, and Sobi. A.R. has received consultancy fees and honoraria from Alexion, AstraZeneca Rare Disease, Roche, and Novartis and research funding from Alexion, AstraZeneca Rare Disease and Roche. J.W.L. has received grants from Alexion, AstraZeneca Rare Disease and Achillion; is a member of an advisory board for and has received honoraria from Alexion, AstraZeneca Rare Disease; and is a consultant to Kyowa Kirin, Novartis, and Sanofi.
Figures

Comment in
-
A belt-and-suspenders approach to PNH.Blood. 2025 Feb 20;145(8):785-786. doi: 10.1182/blood.2024027600. Blood. 2025. PMID: 39976947 No abstract available.
References
-
- Lee JW, Brodsky RA, Nishimura JI, Kulasekararaj AG. The role of the alternative pathway in paroxysmal nocturnal hemoglobinuria and emerging treatments. Expert Rev Clin Pharmacol. 2022;15(7):851–861. - PubMed
-
- Kulasekararaj A, Brodsky R, Griffin M, et al. P812: Long-term complement inhibition and survival outcomes in patients with paroxysmal nocturnal hemoglobinuria: an interim analysis of the ravulizumab clinical trials. HemaSphere. 2022;6:706–707.
-
- Hillmen P, Young NS, Schubert J, et al. The complement inhibitor eculizumab in paroxysmal nocturnal hemoglobinuria. N Engl J Med. 2006;355(12):1233–1243. - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

