Refining management strategies for Lennox-Gastaut syndrome: Updated algorithms and practical approaches
- PMID: 39700524
- PMCID: PMC11803293
- DOI: 10.1002/epi4.13075
Refining management strategies for Lennox-Gastaut syndrome: Updated algorithms and practical approaches
Abstract
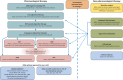
Lennox-Gastaut syndrome (LGS) is a severe developmental and epileptic encephalopathy (DEE) characterized by multiple types of drug-resistant seizures (which must include tonic seizures) with classical onset before 8 years (although some cases with later onset have also been described), abnormal electroencephalographic features, and cognitive and behavioral impairments. Management and treatment of LGS are challenging, due to associated comorbidities and the treatment resistance of seizures. A panel of five epileptologists reconvened to provide updated guidance and treatment algorithms for LGS, incorporating recent advancements in antiseizure medications (ASMs) and understanding of DEEs. The resulting consensus document is based on current evidence from clinical trials and clinical practice and the panel's expert opinion, focusing on new ASMs with novel mechanisms of action, such as highly purified cannabidiol and fenfluramine. For a patient presenting with newly diagnosed LGS or suspected LGS, the recommended first-line treatment continues to be valproate. If this is ineffective as monotherapy, adjunctive therapy with, firstly, lamotrigine and secondly, rufinamide, is recommended. If seizure control remains suboptimal, subsequent adjunctive ASM treatment options include (alphabetically) cannabidiol, clobazam, felbamate, fenfluramine, and topiramate, although evidence for these is more limited. Whenever possible, no more than two ASMs should be used together. Nonpharmacological treatment approaches should be used in conjunction with ASM therapy and include ketogenic diet therapies, vagus nerve stimulation, and corpus callosotomy. Patients with LGS that has evolved from another type of epilepsy who are not already being treated with valproate should be transitioned to valproate and then managed using the same algorithm as for newly diagnosed LGS. Older patients with established LGS should be reviewed at least annually by a suitably experienced neurologist. The revised guidance aims to improve seizure control and quality of life for patients with LGS through personalized, evidence-based treatment strategies while addressing the challenges of accurate diagnosis and management in a rapidly evolving therapeutic landscape. PLAIN LANGUAGE SUMMARY: Lennox-Gastaut syndrome (LGS) is a severe type of epilepsy that usually starts in childhood but continues into adulthood. It is characterized by a variety of different types of seizures (abnormal electrical activity in the brain), which are difficult to treat and often cause people with the condition to fall and injure themselves. Most people with LGS have learning difficulties and need a lot of support, often in residential care. The authors are experts in treating people with LGS and this article provides up-to-date guidance and advice on how best to care for those with the condition.
Keywords: antiseizure medications; developmental and epileptic encephalopathy; epilepsy; therapy algorithm; update.
© 2024 The Author(s). Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
Stéphane Auvin is Deputy Editor for
We confirm that we have read the Journal's position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
References
-
- Specchio N, Wirrell EC, Scheffer IE, Nabbout R, Riney K, Samia P, et al. International league against epilepsy classification and definition of epilepsy syndromes with onset in childhood: position paper by the ILAE task force on nosology and definitions. Epilepsia. 2022;63:1398–1442. - PubMed
-
- European Medicines Agency . Epidyolex (cannabidiol) summary of product characteristics. [Cited 2019 Oct 4]. Available from: https://www.ema.europa.eu/en/documents/product‐information/epidyolex‐epa...
-
- US Food and Drug Administration . Epidiolex (cannabidiol) prescribing information. [Cited 2020 Jun]. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2021/210365Orig1s01...
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

