Phase II Trial of Neoadjuvant Docetaxel/Cisplatin/5-Fluorouracil Combined with Pegteograstim for Unresectable, Locally Advanced Sinonasal Squamous Cell Carcinoma: KCSG HN18-07
- PMID: 39701091
- PMCID: PMC12263236
- DOI: 10.4143/crt.2024.1025
Phase II Trial of Neoadjuvant Docetaxel/Cisplatin/5-Fluorouracil Combined with Pegteograstim for Unresectable, Locally Advanced Sinonasal Squamous Cell Carcinoma: KCSG HN18-07
Abstract
Purpose: The role of neoadjuvant chemotherapy in locally advanced sinonasal squamous cell carcinoma (SNSCC) has not been established prospectively. We conducted a phase II trial of neoadjuvant chemotherapy (NAC) with docetaxel/cisplatin/5-fluorouracil (TPF) in this population.
Materials and methods: Eligible patients had unresectable, locally advanced SNSCC, defined as T3/4 category or potential compromise of critical organ function on surgery. Three TPF (docetaxel 75 mg/m2 and cisplatin 75 mg/m2 on day 1, 5-fluorouracil 1,000 mg/m2 on days 1-4 every 3 weeks) cycles were administered with prophylactic pegteograstim. The primary outcome was the objective response rate (ORR); the secondary outcomes included 2-year progression-free survival (PFS), eyeball preservation rate, and safety.
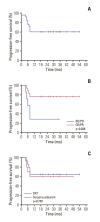
Results: Among 28 patients screened, 25 were evaluable for efficacy (one screen-failure; two evaluable for safety only). The confirmed ORR was 72.0%. The definitive post-NAC treatment comprised chemoradiotherapy (n=15) and surgery (n=10). With a median follow-up of 25.5 months, median PFS was not reached and the 2-year PFS rate was 60.4%. Response to NAC was related to prolonged PFS (p=0.038). No patient underwent eyeball exenteration at the data cutoff point. Treatment-related adverse events of grade ≥ 3 were neutropenia (48.1%) including febrile neutropenia (14.8%), followed by acute kidney injury (22.2%), nausea/vomiting (11.1%), anemia (7.4%), thrombocytopenia (7.4%), and enterocolitis (3.7%).
Conclusion: TPF NAC showed a promising efficacy and might help preserve critical structures in this population, which needs to be validated in a large prospective trial (KCT0003377).
Keywords: Nasal cavity; Neoadjuvant chemotherapy; Paranasal sinus; Prognosiss; Squamous cell carcinoma.
Conflict of interest statement
Pegteograstim was provided by Green Cross Cooperation (Korea).
Figures


References
-
- Ackall FY, Issa K, Barak I, Teitelbaum J, Jang DW, Jung SH, et al. Survival outcomes in sinonasal poorly differentiated squamous cell carcinoma. Laryngoscope. 2021;131:E1040–8. - PubMed
-
- Dutta R, Dubal PM, Svider PF, Liu JK, Baredes S, Eloy JA. Sinonasal malignancies: a population-based analysis of site-specific incidence and survival. Laryngoscope. 2015;125:2491–7. - PubMed
-
- Lee MM, Vokes EE, Rosen A, Witt ME, Weichselbaum RR, Haraf DJ. Multimodality therapy in advanced paranasal sinus carcinoma: superior long-term results. Cancer J Sci Am. 1999;5:219–23. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

