Artemether-lumefantrine-amodiaquine or artesunate-amodiaquine combined with single low-dose primaquine to reduce Plasmodium falciparum malaria transmission in Ouélessébougou, Mali: a five-arm, phase 2, single-blind, randomised controlled trial
- PMID: 39701119
- PMCID: PMC11798902
- DOI: 10.1016/j.lanmic.2024.100966
Artemether-lumefantrine-amodiaquine or artesunate-amodiaquine combined with single low-dose primaquine to reduce Plasmodium falciparum malaria transmission in Ouélessébougou, Mali: a five-arm, phase 2, single-blind, randomised controlled trial
Abstract
Background: Triple artemisinin-based combination therapies (TACTs) can delay the spread of antimalarial drug resistance. Artesunate-amodiaquine is widely used for uncomplicated Plasmodium falciparum malaria. We therefore aimed to determine the safety and efficacy of artemether-lumefantrine-amodiaquine and artesunate-amodiaquine with and without single low-dose primaquine for reducing gametocyte carriage and transmission to mosquitoes.
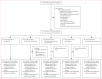
Methods: We did a five-arm, single-blind, phase 2 randomised controlled trial at the Ouélessébougou Clinical Research Unit of the Malaria Research and Training Centre of the University of Sciences, Techniques and Technologies of Bamako in Mali. Eligible participants were aged 10-50 years, with asymptomatic P falciparum microscopy-detected gametocyte carriage. Eligible participants were randomly allocated (1:1:1:1:1) to receive either artemether-lumefantrine, artemether-lumefantrine-amodiaquine, artemether-lumefantrine-amodiaquine plus primaquine, artesunate-amodiaquine, or artesunate-amodiaquine plus primaquine. Treatment regimens were administered on days 0, 1, and 2; primaquine was given as a single dose on day 0. All staff except the trial pharmacist and participants were masked to the treatment allocation. The primary outcome was the median percentage change in mosquito infection rate between pretreatment and 2 days after treatment initiation, assessed by direct membrane feeding assay. Data were analysed using a per-protocol analysis. This study is registered with ClinicalTrials.gov, NCT05550909.
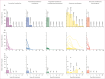
Findings: Between Oct 16, 2022, and Dec 28, 2022, a total of 1249 individuals were screened; of whom, 100 were enrolled and randomly assigned to one of the five treatment groups (20 per group). Before treatment, 61 (61%) of 100 participants were infectious to mosquitoes, with a median of 7·3% (IQR 3·2 to 23·5) of mosquitoes becoming infected. Among infectious participants, the median percentage reduction in mosquito infection rate between pretreatment and 2 days after treatment was 100% (IQR 100 to 100) in the artemether-lumefantrine (p=0·0018), artemether-lumefantrine-amodiaquine (p=0·0018), and artemether-lumefantrine-amodiaquine plus primaquine (p=0·0009) treatment groups. In the artesunate-amodiaquine group the median percentage reduction in mosquito infection rate was only 32% (IQR -10·9 to 79·4; p=0·19), whereas a 100% median reduction was seen in the artesunate-amodiaquine plus primaquine group (IQR 100 to 100; p=0·0009). At day 2, two (10%) of 20 participants in the artemether-lumefantrine group, two (11%) of 19 in the artemether-lumefantrine-amodiaquine group, and 15 (75%) of 20 in the artesunate-amodiaquine group infected any number of mosquitoes whereas no infected mosquitoes were observed at this timepoint in the groups with primaquine. 85 (85%) of 100 participants had a total of 262 adverse events during follow-up; of which, 181 (69%) were categorised as mild and 81 (31%) as moderate. No serious adverse events were reported.
Interpretation: Our findings support the effectiveness of artemether-lumefantrine alone or as part of TACT for preventing nearly all human-mosquito malaria parasite transmission within 48 h. By contrast, substantial transmission was observed following treatment with artesunate-amodiaquine. The addition of a single low dose of primaquine blocks transmission to mosquitoes rapidly regardless of schizonticide.
Funding: Bill & Melinda Gates Foundation.
Copyright © 2024 The Author(s). Published by Elsevier Ltd.. All rights reserved.
Conflict of interest statement
Declaration of interests We declare no competing interests.
Figures
References
-
- WHO . World Health Organization; Geneva: 2023. World malaria report 2023.https://www.who.int/teams/global-malaria-programme/reports/world-malaria...
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials

