Lisdexamfetamine in the treatment of methamphetamine dependence: A randomised, placebo-controlled trial
- PMID: 39701142
- PMCID: PMC12128569
- DOI: 10.1111/add.16730
Lisdexamfetamine in the treatment of methamphetamine dependence: A randomised, placebo-controlled trial
Abstract
Aims: This study tested the efficacy and safety of a 12-week course of lisdexamfetamine in reducing methamphetamine use, an outcome which is associated with improvements in health and wellbeing, in people dependent on methamphetamine.
Design, setting and participants: This study was a randomised double-blind placebo-controlled trial conducted in six specialist outpatient clinics in Adelaide, Melbourne, Newcastle and Sydney, Australia (2018-2021). Participants were164 adults with methamphetamine dependence, reporting at least 14 use days out of the previous 28 days (62% male, 38% female, < 1% other; mean age 39 years).
Interventions: Participants were randomly allocated 1:1 to a 15-week regimen of lisdexamfetamine (1-week induction to 250 mg, 12-week maintenance regimen, 2-week reduction; n = 80) or matched placebo (n = 84), followed-up to Week 19.
Measurements: The primary efficacy measure was past 28-day methamphetamine use at Week 13. Safety was assessed by adverse event rates. Secondary measures included methamphetamine use during the 12-week treatment period and treatment satisfaction.
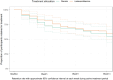
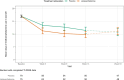
Findings: Nine randomized participants did not start treatment (five were allocated to lisdexamfetamine and four allocated to placebo) and were excluded from the analyses. Fifty-seven per cent of participants were retained on study medication to primary end-point. There was only weak evidence of a lisdexamfetamine benefit at 13 weeks [adjusted difference in days of methamphetamine use = 2.2, 95% confidence interval (CI) = -0.5 to 5.0; P = 0.49]. However, throughout the whole 12-week treatment maintenance phase, the lisdexamfetamine group had fewer days of methamphetamine use in total (difference = 8.8, 95% CI = 2.7-15.0; P = 0.005). The lisdexamfetamine group reported greater self-reported treatment effectiveness [odds ratio (OR) = 2.89, 95% CI = 1.67-5.02; P < 0.001] and treatment satisfaction (OR = 3.80, 95% CI = 1.93-7.47; P < 0.001). Adverse events with lisdexamfetamine included nausea. Serious adverse events occurred in four (5%) of participants who received lisdexamfetamine.
Conclusions: Lisdexamfetamine appears to reduce methamphetamine use over a 12-week treatment period, although there is only weak evidence that reduced use is maintained during the last 4 weeks.
Keywords: Clinical trial; lisdexamfetamine; methamphetamine; methamphetamine dependence; methamphetamine use disorder; non‐abstinence outcomes; randomized controlled trial; stimulants.
© 2024 The Author(s). Addiction published by John Wiley & Sons Ltd on behalf of Society for the Study of Addiction.
Conflict of interest statement
D.L. is supported by a NHMRC Investigator Grant (1196892). M.F. has received unrestricted funding for research purposes from Indivior and Sequiiris. M.M. has been an advisory board member for AbbVie Australia and Pfizer Australia, both unrelated to this project. N.L. has received funding from NHMRC, Camurus AB and Indivior for unrelated research. R.B. was an investigator on an untied education grant from Mundipharma to conduct post‐marketing surveillance on oxycodone and an untied educational grant from Reckitt‐Benckiser to develop a scale to identify extra medical use of pharmaceutical opioids. S.A. has received speaker honoraria from Camurus, Indivior, Gilead and Janssen for work unrelated to this study. All other authors have no relevant conflicts to declare.
Figures
References
-
- United Nations Office on drugs and crime . World Drug Report 2023. Available at: https://www.unodc.org/unodc/en/data-and-analysis/world-drug-report-2023..... Accessed 26 February 2024.
-
- Hillhouse MP, Marinelli‐Casey P, Gonzales R, Ang A, Rawson RA, Authors MTPC. Predicting in‐treatment performance and post‐treatment outcomes in methamphetamine users. Addiction. 2007;96:84–95. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous