Early initiation of intravenous cyclophosphamide and one-year outcome in super-refractory cryptogenic-new onset refractory status epilepticus
- PMID: 39701580
- PMCID: PMC11803287
- DOI: 10.1002/epi4.13055
Early initiation of intravenous cyclophosphamide and one-year outcome in super-refractory cryptogenic-new onset refractory status epilepticus
Abstract
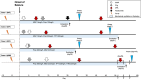
To explore the potential efficacy of early initiation of intravenous cyclophosphamide (IVCPA), we reviewed consecutive four cases of super-refractory cryptogenic-new onset refractory status epilepticus (C-NORSE) between 2015 and 2023. We compared functional outcomes at 3 months and 1 year after the onset between patients who received IVCPA within 20 days (early-treated) and those who received it later (late-treated). All patients (median age: 43 years) had a prodromal fever. Brain MRI revealed symmetrically increased FLAIR signals in the medial temporal lobes of all patients. Despite initiating antiseizure medications (ASMs) and first-line immunotherapy (intravenous-methylprednisolone and immunoglobulins) within a median of 3 days from onset, SE persisted >5 days. The diagnosis of C-NORSE was suggested based on a high C-NORSE score (6/6). Thus, all patients received IVCPA a median of 15.5 days after seizure onset (three within 20 days and one at 31 days). One of the three early-treated patients also received tocilizumab. Early-treated patients exhibited shorter sedation periods (median 29 vs. 75 days) and better 1 year functional status (mRS 1-2 vs. mRS 4) compared to the late-treated patient. Early initiation of IVCPA and/or tocilizumab, along with ASMs, may contribute to a better one-year functional status in super-refractory C-NORSE patients. PLAIN LANGUAGE SUMMARY: This study demonstrates the potential efficacy of early administration of intravenous cyclophosphamide on one-year functional status in patients with super-refractory cryptogenic-new onset refractory status epilepticus. "Early-treated patients" who received it within 20 days of seizure onset achieved a good one-year functional status. The "late-treated patient" (Case 4) who received it later did not achieve a good functional status. Early initiation of cyclophosphamide, along with antiseizure medications, may contribute to a better one-year functional status in this population.
Keywords: NORSE; autoantibodies; brain MRI; immunotherapy.
© 2024 The Author(s). Epilepsia Open published by Wiley Periodicals LLC on behalf of International League Against Epilepsy.
Conflict of interest statement
The authors declare that they have no conflicts of interest. We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
Figures
References
-
- Wilder‐Smith EP, Lim EC, Teoh HL, Sharma VK, Tan JJH, Chan BPL, et al. The NORSE (new‐onset refractory status epilepticus) syndrome: defining a disease entity. Ann Acad Med Singapore. 2005;34:417–420. - PubMed
-
- Sculier C, Gaspard N. New onset refractory status epilepticus (NORSE). Seizure. 2019;68:72–78. - PubMed
-
- Hirsch LJ, Gaspard N, van Baalen A, Nabbout R, Demeret S, Loddenkemper T, et al. Proposed consensus definitions for new‐onset refractory status epilepticus (NORSE), febrile infection‐related epilepsy syndrome (FIRES), and related conditions. Epilepsia. 2018;59:739–744. - PubMed
-
- Wickstrom R, Taraschenko O, Dilena R, Payne ET, Specchio N, Nabbout R, et al. International consensus recommendations for management of new onset refractory status epilepticus (NORSE) incl. Febrile infection‐related epilepsy syndrome (FIRES): statements and supporting evidence. Epilepsia. 2022;63:2840–2864. - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

