Somatic PTEN and ARID1A loss and endometriosis disease burden: a longitudinal study
- PMID: 39701665
- PMCID: PMC11788214
- DOI: 10.1093/humrep/deae269
Somatic PTEN and ARID1A loss and endometriosis disease burden: a longitudinal study
Abstract
Study question: Is there an association between the somatic loss of PTEN (phosphatase and tensin homolog) and ARID1A (AT-rich interaction domain 1A) and endometriosis disease severity and worse clinical outcomes?
Summary answer: Somatic PTEN loss in endometriosis epithelium was associated with greater disease burden and subsequent surgical complexity.
What is known already: Somatic cancer-driver mutations including those involving the PTEN and ARID1A genes exist in endometriosis without cancer; however, their clinical impact remains unclear.
Study design, size, duration: This prospective longitudinal study involved endometriosis tissue and clinical data from 126 participants who underwent surgery at a tertiary center for endometriosis (2013-2017), with a follow-up period of 5-9 years.
Participants/materials, setting, methods: PTEN and ARID1A loss was assessed using established immunohistochemistry (IHC) methods as proxies for somatic loss by two independent raters. PTEN and ARID1A status for each participant was defined as loss (loss in at least one sample for a participant) or retained (no loss in all samples for a participant). Primary analyses examined associations between PTEN and ARID1A loss and disease burden based on anatomic subtype (superficial peritoneal endometriosis (SUP), deep endometriosis (DE), ovarian endometrioma (OMA)) and rASRM stage (I-IV). Secondary analyses explored associations of PTEN and ARID1A loss with demographics, surgical difficulty, and pain scores (baseline and follow-up). Additionally, using previously published data on KRAS codon 12 mutations for this cohort, we investigated associations between variables in the primary and secondary analyses and acquiring two or more somatic events (PTEN loss, ARID1A loss, or KRAS mutation) in this cohort. The risk of reoperation over the 5-9 years was also examined.
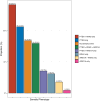
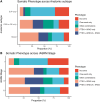
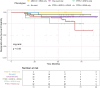
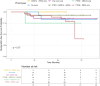
Main results and the role of chance: PTEN loss (68.3%; 86 participants) exceeded ARID1A loss (24.6%; 31 participants). Inter-rater reliability was substantial for PTEN (k = 0.69; 95% CI: 0.62-0.77) and ARID1A (k = 0.64; 95% CI: 0.51-0.77). PTEN loss was significantly associated with more severe anatomic subtypes (P < 0.001; participants with SUP only = 46.4%; participants with DE only or OMA only = 72.7%; participants with mixed subtypes = 85.1%), and higher stages (P = 0.024; Stage I = 47.8%; Stage II = 73.7%; Stage III = 80.8%; Stage IV = 81.0%). Results were similar for ARID1A loss, albeit with smaller sample size limiting power. PTEN loss was further associated with non-White ethnicities (P = 0.017) and greater surgical difficulty (more frequent need for ureterolysis) (P = 0.02). There were no differences in pain scores (baseline or follow-up) based on PTEN or ARID1A status. Reoperation was uncommon (13.5% of the cohort), and patterns in reoperation rates based on the presence of somatic alterations did not reach statistical significance.
Limitations, reasons for caution: Sequencing was not performed to determine the type of PTEN and ARID1A somatic mutations resulting in loss of expression.
Wider implications of the findings: These results demonstrate a link between PTEN somatic loss and greater endometriosis disease burden. These findings underscore the potential relevance of PTEN loss and other somatic driver mutations in a future molecular classification of endometriosis.
Study funding/competing interest(s): This study was funded by Canadian Institutes of Health Research (CIHR) project grant (MOP-142273 and PJT-156084). P.J.Y. was supported by a Health Professional Investigator award from Michael Smith Health Research BC, Canada, and a Canada Research Chair (Tier 2) in Endometriosis and Pelvic Pain. M.S.A. was supported by a Michael Smith Health Research BC Scholar award, and CIHR project grants (369990, 462997, and 456767). The sponsors did not play any role in the study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the article for publication. C.A. declares receiving payment from Pfizer for a symposium; being on advisory boards for AbbVie and Pfizer; being President and past President of the Canadian Society for the Advancement of Gynecologic Excellence (CanSAGE), co-lead of EndoAct Canada, and a board member of IPPS. M.A.B. has received consulting fees from AbbVie and Pfizer and grants from Ferring outside the scope of this work. D.G.H. is the founder of Canxeia Health but has no current affiliation. M.K. has received consulting fees from Helix Biopharma outside the scope of this work. M.S.A. received reimbursement of travel and registration fees to attend and present at the 2023 and 2024 annual meetings for the Society for Reproductive Investigation (SRI). P.J.Y. declares receiving: payment for a lecture from the International Society for the Study of Women's Sexual Health (ISSWSH); honoraria from the CIHR; support to attend meetings from CanSAGE, ISSWSH, the International Pelvic Pain Society, the World Endometriosis Society (WES), the Society for the Study of Reproduction, and the Vulvodynia Summit; and discounted devices from Ohnut Wearable for a clinical trial. P.J.Y. is a data safety monitoring board member of a clinical trial funded by CIHR; and a strategic advisory board member for the Women's Health Research Institute. P.J.Y. served as a board of directors member for CanSAGE and ISSWSH; was a junior board of directors member for WES; is a current board of directors member for WES; and was a committee chair for the Society of Obstetricians and Gynaecologists of Canada. A subset of these results was presented by the first author at the 71st Society for Reproductive Investigation Annual Scientific Meeting on 15 March 2024. Other authors have nothing to declare.
Trial registration number: N/A.
Keywords: KRAS; ARID1A; PTEN; endometriosis; mutation; somatic events.
© The Author(s) 2024. Published by Oxford University Press on behalf of European Society of Human Reproduction and Embryology.
Conflict of interest statement
C.A. declares receiving payment from Pfizer for a symposium; being on advisory boards for AbbVie and Pfizer; being President and past President of the Canadian Society for the Advancement of Gynecologic Excellence (CanSAGE), co-lead of EndoAct Canada, and a board member of IPPS. M.A.B. has received consulting fees from AbbVie and Pfizer and grants from Ferring outside the scope of this work. D.G.H. is the founder of Canxeia Health but has no current affiliation. M.K. has received consulting fees from Helix Biopharma outside the scope of this work. M.S.A. received reimbursement of travel and registration fees to attend and present at the 2023 and 2024 annual meetings for the Society for Reproductive Investigation (SRI). P.J.Y. declares receiving: payment for a lecture from the International Society for the Study of Women’s Sexual Health (ISSWSH); honoraria from the CIHR; support to attend meetings from CanSAGE, ISSWSH, the International Pelvic Pain Society, the World Endometriosis Society (WES), the Society for the Study of Reproduction, and the Vulvodynia Summit; and discounted devices from Ohnut Wearable for a clinical trial. P.J.Y. is a data safety monitoring board member of a clinical trial funded by CIHR; and a strategic advisory board member for the Women’s Health Research Institute. P.J.Y. served as a board of directors member for CanSAGE and ISSWSH; was a junior board of directors member for WES; is a current board of directors member for WES; and was a committee chair for the Society of Obstetricians and Gynaecologists of Canada. A subset of these results was presented by the first author at the 71st Society for Reproductive Investigation Annual Scientific Meeting on 15 March 2024. Other authors have nothing to declare.
Figures

Similar articles
-
Iatrogenic endometriosis harbors somatic cancer-driver mutations.Hum Reprod. 2019 Jan 1;34(1):69-78. doi: 10.1093/humrep/dey332. Hum Reprod. 2019. PMID: 30428062 Free PMC article.
-
ESHRE guideline: endometriosis.Hum Reprod Open. 2022 Feb 26;2022(2):hoac009. doi: 10.1093/hropen/hoac009. eCollection 2022. Hum Reprod Open. 2022. PMID: 35350465 Free PMC article.
-
Endometriosis classification systems: an international survey to map current knowledge and uptake.Hum Reprod Open. 2022 Mar 1;2022(1):hoac002. doi: 10.1093/hropen/hoac002. eCollection 2022. Hum Reprod Open. 2022. PMID: 35237731 Free PMC article.
-
No additional risk of congenital anomalies after first-trimester dydrogesterone use: a systematic review and meta-analysis.Hum Reprod Open. 2024 Jan 23;2024(1):hoae004. doi: 10.1093/hropen/hoae004. eCollection 2024. Hum Reprod Open. 2024. PMID: 38344249 Free PMC article. Review.
-
Current global status of male reproductive health.Hum Reprod Open. 2024 Apr 12;2024(2):hoae017. doi: 10.1093/hropen/hoae017. eCollection 2024. Hum Reprod Open. 2024. PMID: 38699533 Free PMC article. Review.
References
-
- Allaire C, Williams C, Bodmer-Roy S, Zhu S, Arion K, Ambacher K, Wu J, Yosef A, Wong F, Noga H et al. Chronic pelvic pain in an interdisciplinary setting: 1-year prospective cohort. Am J Obstet Gynecol 2018;218:114.e1–114.e12. - PubMed
-
- Álvarez-Garcia V, Tawil Y, Wise HM, Leslie NR. Mechanisms of PTEN loss in cancer: it’s all about diversity. Semin Cancer Biol 2019;59:66–79. - PubMed
-
- Anglesio MS, Yong PJ. Endometriosis-associated ovarian cancers. Clin Obstet Gynecol 2017;60:711–727. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

