Persistence of advanced therapies in patients with inflammatory bowel disease: retrospective cohort study using a large healthcare claims database in Japan
- PMID: 39701922
- PMCID: PMC12332283
- DOI: 10.5217/ir.2024.00118
Persistence of advanced therapies in patients with inflammatory bowel disease: retrospective cohort study using a large healthcare claims database in Japan
Abstract
Background/aims: There are few studies that comprehensively report real-world persistence for first-line advanced therapies used to treat inflammatory bowel disease. We aimed to describe persistence of first-line advanced therapies among incident biologic or Janus kinase inhibitor users with inflammatory bowel disease.
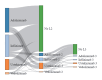
Methods: Retrospective cohort study using the Japan Medical Data Center database from January 1, 2010, until September 30, 2022. Patients aged ≥15 years with relevant diagnostic and treatment codes were included. All eligible patients were observed until study end (September 30, 2022), death, or disenrollment, whichever occurred first.
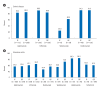
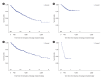
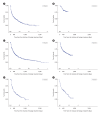
Results: Among 1,115 patients with Crohn's disease included in the analysis, 41.4% initiated adalimumab, 37.4% infliximab, 18.1% ustekinumab, and 3.0% vedolizumab. Median age was 31.2-34.8 years, 72.8% to 85.9% were male. Persistence at 12 months was 84.7% for adalimumab, 87.7% for infliximab, 91.3% for ustekinumab, and 53.1% for vedolizumab. Persistence at 24 months was 76.3%, 76.8%, 80.4%, and 28.6%, respectively. Among 1,942 patients with ulcerative colitis, 24.8% initiated adalimumab, 33.6% infliximab, 11.2% golimumab, 17.5% vedolizumab, 5.6% ustekinumab, and 7.3% tofacitinib. Mean age was 38.2-40.4 years, 57.4% to 65.8% were male. Persistence at 12 months was 57.6% for adalimumab, 87.7% for infliximab, 54.9% for golimumab, 69.7% for vedolizumab, and 84.0% for ustekinumab. At month 24, persistence for ustekinumab was 75.0%, versus 42.9%-59.4% for other treatments.
Conclusions: Index treatment with ustekinumab resulted in high persistence through 24 months after initiation in patients with Crohn's disease or ulcerative colitis. Our study provides insights into the real-world usage of advanced treatments for patients with IBD in Japan.
Keywords: Crohn disease; Inflammatory bowel disease; Persistence; Ulcerative colitis; Ustekinumab.
Conflict of interest statement
Matsuoka K has received a research grant from Janssen Pharmaceutical K.K. outside the scope of this work; has received scholarship grants from AbbVie, EA Pharma, JIMRO, Kissei, Kyorin, Mitsubishi Tanabe, Mochida, Nippon Kayaku; and has received honoraria from AbbVie, Celltrion Healthcare, EA Pharma, Eli Lilly, Gilead Sciences, Janssen Pharmaceutical K.K., JIMRO, Kissei, Kyorin, Mitsubishi Tanabe, Miyarisan, Mochida, Nippon Kayaku, Pfizer, Takeda, Zeria. Kawamura S, Zhang Y, Wahking B, Tan JY, and Qiu H are employees of Johnson & Johnson and hold stock/shares in Johnson & Johnson. Nakajo K and Chung H are employees of Johnson & Johnson.
Matsuoka K is an editorial board member of the journal but was not involved in the peer reviewer selection, evaluation, or decision process of this article. No other potential conflicts of interest relevant to this article were reported.
Figures
References
-
- Gajendran M, Loganathan P, Catinella AP, Hashash JG. A comprehensive review and update on Crohn’s disease. Dis Mon. 2018;64:20–57. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

