Denatonium inhibits RANKL-induced osteoclast differentiation and rescues the osteoporotic phenotype by blocking p65 signaling pathway
- PMID: 39701944
- PMCID: PMC11660935
- DOI: 10.1186/s10020-024-01031-2
Denatonium inhibits RANKL-induced osteoclast differentiation and rescues the osteoporotic phenotype by blocking p65 signaling pathway
Abstract
Background: Bone remodeling is a critical process that maintains skeletal integrity, orchestrated by the balanced activities of osteoclasts, which resorb bone, and osteoblasts, which form bone. Osteoclastogenesis, the formation of osteoclasts, is primarily driven by NFATc1, a process activated through c-Fos and NF-κB signaling pathways in response to receptor activator of nuclear factor κB ligand (RANKL). Dysregulation of RANKL signaling is a key contributor to pathological bone loss, as seen in conditions such as osteoporosis.
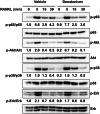
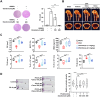
Methods: We investigated the effects of denatonium, a known bitter compound, on RANKL-induced osteoclast differentiation. We used RNA sequencing (RNA-seq) to analyze gene expression profiles in osteoclast precursors treated with denatonium. Transcription factor prediction analysis was conducted to identify key targets of denatonium action. Additionally, we performed Western blotting to examine the phosphorylation status of AKT and p65, crucial components of the NF-κB pathway. Chromatin immunoprecipitation (ChIP) assays were employed to assess the binding of p65 to promoter regions of osteoclast-related genes. Finally, we tested the therapeutic potential of denatonium in a mouse model of osteoporosis.
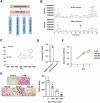
Results: Our findings demonstrated that denatonium significantly inhibited RANKL-induced osteoclastogenesis by targeting the p65 pathway. RNA-seq analysis revealed a downregulation of osteoclast-related genes following denatonium treatment, corroborated by transcription factor prediction analysis, which highlighted p65 as a key target. Denatonium effectively blocked the phosphorylation of AKT and p65, key steps in NF-κB activation. ChIP assays further confirmed that denatonium reduced the enrichment of p65 at promoter regions critical for osteoclast differentiation. In vivo, denatonium treatment in an osteoporosis animal model led to a significant restoration of bone health, demonstrating its potential as a therapeutic agent.
Conclusions: This study identifies denatonium as an inhibitor of RANKL-induced osteoclast differentiation, potentially acting through suppression of the p65 signaling pathway. The ability of denatonium to downregulate osteoclast-related genes and inhibit key signaling events highlights its potential as a candidate for further investigation in the context of bone loss and osteoporosis.
Keywords: Denatonium; Osteocastogenesis; Osteoporosis; p65.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Institutional Animal Management and Use Committee of Chungbuk National University (CBNUA-1245-19-02 and CBNUA-1391-20-01). Consent for publication: All the authors have read this manuscript and would like to have it considered exclusively for publication. Competing interests: The authors declare no competing interests.
Figures


References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

