The GRAS gene family and its roles in pineapple (Ananas comosus L.) developmental regulation and cold tolerance
- PMID: 39701971
- PMCID: PMC11657692
- DOI: 10.1186/s12870-024-05913-9
The GRAS gene family and its roles in pineapple (Ananas comosus L.) developmental regulation and cold tolerance
Abstract
Background: Pineapple (Ananas comosus L.) is a major tropical fruit crop with considerable economic importance, and its growth and development are significantly impacted by low temperatures. The plant-specific GRAS gene family plays crucial roles in diverse processes, including flower and fruit development, as well as in stress responses. However, the role of the GRAS family in pineapple has not yet been systematically analyzed.
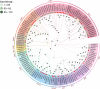
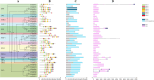
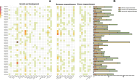
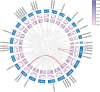
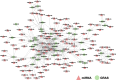
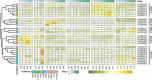
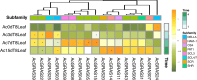
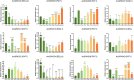
Results: In this study, 43 AcGRAS genes were identified in the pineapple genome; these genes were distributed unevenly across 19 chromosomes and 6 scaffolds and were designated as AcGRAS01 to AcGRAS43 based on their chromosomal locations. Phylogenetic analysis classified these genes into 14 subfamilies: OS19, HAM-1, HAM-2, SCL4/7, LISCL, SHR, PAT1, DLT, LAS, SCR, SCL3, OS43, OS4, and DELLA. Gene structure analysis revealed that 60.5% of the AcGRAS genes lacked introns. Expression profiling demonstrated tissue-specific expression, with most AcGRAS genes predominantly expressed in specific floral organs, fruit tissues, or during particular developmental stages, suggesting functional diversity in pineapple development. Furthermore, the majority of AcGRAS genes were induced by cold stress, but different members seemed to play distinct roles in short-term or long-term cold adaptation in pineapple. Notably, most members of the PAT1 subfamily were preferentially expressed during late petal development and were upregulated under cold stress, suggesting their special roles in petal development and the cold response. In contrast, no consistent expression patterns were observed among genes in other subfamilies, suggesting that various regulatory factors, such as miRNAs, transcription factors, and cis-regulatory elements, may contribute to the diverse functions of AcGRAS members, even within the same subfamily.
Conclusions: This study provides the first comprehensive analysis of GRAS genes in pineapple, offers valuable insights for further functional investigations of AcGRASs and provides clues for improving pineapple cold resistance breeding.
Keywords: GRAS transcription factor; Gene expression; Genome-wide analysis; Pineapple.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: The experimental research and method on pineapple species comply with relevant institutional, national, and international guidelines. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures



References
-
- Gonzalez DH. Plant transcription factors: evolutionary, structural and functional aspects. London: Academic Press; 2015.
-
- Liu X, Widmer A. Genome-wide comparative analysis of the GRAS gene family in Populus, Arabidopsis and rice. Plant Mol Biol Repo. 2014;32:1129–45. - DOI
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

