Revealing the key role of the folate synthesis regulatory gene DHNA in tobacco leaf yellowing based on BSA-seq, RNA-seq, and proteomic sequencing
- PMID: 39701982
- PMCID: PMC11656980
- DOI: 10.1186/s12870-024-05917-5
Revealing the key role of the folate synthesis regulatory gene DHNA in tobacco leaf yellowing based on BSA-seq, RNA-seq, and proteomic sequencing
Abstract
Background: The degree of yellowing in tobacco leaves is an important indicator for determining the maturity and harvesting time of tobacco leaves. Decreasing chlorophyll levels helps speed up the ripening process of tobacco leaves for easier mechanical harvesting. Identifying and utilizing genes that regulate yellowing in tobacco leaves are crucial for developing tobacco varieties suitable for mechanized harvesting and understanding the molecular processes that control leaf color changes.
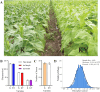
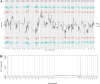
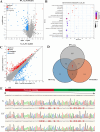
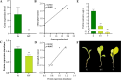
Results: In this study, the phenotypes of the yellow-leaf K326 and K326 varieties were analysed, and it was observed that the yellow-leaf K326 variety exhibited a distinct yellow leaf phenotype with a significant reduction in chlorophyll content. Subsequently, using a combination of BSA-seq, transcriptomic sequencing (RNA-seq), and proteomic sequencing approaches, we identified the candidate gene Nitab4.5_0008674g0010 that encodes dihydroneopterin aldolase as a factor associated with tobacco leaf yellowing. Finally, by measuring the folate content in K326 and Huangye K326, the folate content in Huangye K326 was observed to be significantly lower than that in K326, thus indicating that folate synthesis plays a crucial role in phenotypic changes in tobacco yellow leaves.
Conclusions: This study is the first to use BSA-seq combined with RNA-seq and proteomic sequencing to identify candidate genes in tobacco yellow leaves. The results provide a theoretical basis for the analysis of the mechanism of tobacco yellow leaf mutations.
Keywords: BSA-seq; Chlorophyll content; Leaf yellowing; Proteome; RNA-seq; Tobacco.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Not applicable. Consent for publication: Not applicable. Competing interests: The authors declare no competing interests.
Figures

Similar articles
-
Conjunctive BSA-Seq and BSR-Seq to Map the Genes of Yellow Leaf Mutations in Hot Peppers (Capsicum annuum L.).Genes (Basel). 2024 Aug 23;15(9):1115. doi: 10.3390/genes15091115. Genes (Basel). 2024. PMID: 39336705 Free PMC article.
-
Cytological, Physiological, and Transcriptome Analysis of Leaf-Yellowing Mutant in Camellia chekiangoleosa.Int J Mol Sci. 2024 Dec 27;26(1):132. doi: 10.3390/ijms26010132. Int J Mol Sci. 2024. PMID: 39795989 Free PMC article.
-
Double mutation of two homologous genes YL1 and YL2 results in a leaf yellowing phenotype in soybean [Glycine max (L.) Merr].Plant Mol Biol. 2020 Jul;103(4-5):527-543. doi: 10.1007/s11103-020-01008-9. Epub 2020 Apr 23. Plant Mol Biol. 2020. PMID: 32323129
-
Identifying key genes involved in yellow leaf variation in 'Menghai Huangye' based on biochemical and transcriptomic analysis.Funct Integr Genomics. 2022 Apr;22(2):251-260. doi: 10.1007/s10142-022-00829-9. Epub 2022 Feb 25. Funct Integr Genomics. 2022. PMID: 35211836
-
The control of chlorophyll catabolism and the status of yellowing as a biomarker of leaf senescence.Plant Biol (Stuttg). 2008 Sep;10 Suppl 1:4-14. doi: 10.1111/j.1438-8677.2008.00081.x. Plant Biol (Stuttg). 2008. PMID: 18721307 Review.
Cited by
-
Genome and transcriptome wide association study identify candidate genes regulating folate levels in maize.Front Plant Sci. 2025 Jun 19;16:1606220. doi: 10.3389/fpls.2025.1606220. eCollection 2025. Front Plant Sci. 2025. PMID: 40612597 Free PMC article.
References
-
- Nie R, Wei X, Jin N, Su S, Chen X. Response of photosynthetic pigments, gas exchange and chlorophyll fluorescence parameters to light quality in Phoebe bournei seedlings. Plant Growth Regul. 2024. p. 1–13.
-
- Deng L, Qin P, Liu Z, Wang G, Chen W, Tong J, Xiao L, Tu B, Sun Y, Yan W, et al. Characterization and fine-mapping of a novel premature leaf senescence mutant yellow leaf and dwarf 1 in rice. Plant Physiol Bioch. 2017;111:50–8. - PubMed
-
- Hong-Bing Z, Hui-Jun G, Lin-Shu Z, Jia-Yu GU, Shi-Rong Z, Jun-Hui LI, Lu-Xiang L. Agronomic traits and photosynthetic characteristics of chlorophyll-deficient wheat mutant induced by spaceflight environment. Acta Agronom Sin. 2011;37(1):119–26.
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

