Dietary methionine supplementation promotes mice hematopoiesis after irradiation
- PMID: 39702305
- PMCID: PMC11660814
- DOI: 10.1186/s40779-024-00584-x
Dietary methionine supplementation promotes mice hematopoiesis after irradiation
Abstract
Background: With the increasing risk of nuclear exposure, more attention has been paid to the prevention and treatment of acute radiation syndrome (ARS). Although amino acids are key nutrients involved in hematopoietic regulation, the impacts of amino acids on bone marrow hematopoiesis following irradiation and the associated mechanisms have not been fully elucidated. Hence, it is of paramount importance to study the changes in amino acid metabolism after irradiation and their effects on hematopoiesis as well as the related mechanisms.
Methods: The content of serum amino acids was analyzed using metabolomic sequencing. The survival rate and body weight of the irradiated mice were detected after altering the methionine content in the diet. Extracellular matrix (ECM) protein analysis was performed via proteomics analysis. Inflammatory factors were examined by enzyme-linked immunosorbent assay (ELISA). Flow cytometry, Western blotting, and immunofluorescence were employed to determine the mechanism by which S100 calcium-binding protein A4 (S100A4) regulates macrophage polarization.
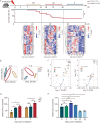
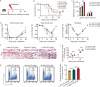
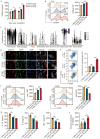
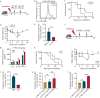
Results: The survival time of irradiated mice was significantly associated with alterations in multiple amino acids, particularly methionine. A high methionine diet promoted irradiation tolerance, especially in the recovery of bone marrow hematopoiesis, yet with dose limitations. Folate metabolism could partially alleviate the dose bottleneck by reducing the accumulation of homocysteine. Mechanistically, high methionine levels maintained the abundance of ECM components, including collagens and glycoproteins, in the bone marrow post-irradiation, among which the level of S100A4 was significantly changed. S100A4 regulated macrophage polarization via the STAT3 pathway, inhibited bone marrow inflammation and facilitated the proliferation and differentiation of hematopoietic stem/progenitor cells.
Conclusions: We have demonstrated that an appropriate elevation in dietary methionine enhances irradiation tolerance in mice and explains the mechanism by which methionine regulates bone marrow hematopoiesis after irradiation.
Keywords: Bone marrow hematopoiesis; Irradiation; Macrophage; Methionine; S100A4.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: This study was approved by the Laboratory Animal Welfare and Ethics Committee of the Army Medical University (AMUWEC2020122, AMUWEC2020055, AMUWEC20211415, AMUWEC20224721). All experiments involving animals were performed following relevant policies. Consent for publication: No applicable. Competing interests: The authors declare that they have no competing financial interests.
Figures




References
MeSH terms
Substances
Grants and funding
- No. 82020108025/International Cooperation and Exchange Programme
- No. 82022061/National Outstanding Youth Foundation of China
- cstc2021jcyj-jqX0004/Natural Science Foundation of Chongqing Municipality
- cstb2022nscq-msx0179/Natural Science Foundation of Chongqing Municipality
- 2023XKRC008/Chongqing Xinqiao Hospital, Second Affiliated Hospital of Army Medical University
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

