Use of equine H3N8 hemagglutinin as a broadly protective influenza vaccine immunogen
- PMID: 39702334
- PMCID: PMC11659547
- DOI: 10.1038/s41541-024-01037-1
Use of equine H3N8 hemagglutinin as a broadly protective influenza vaccine immunogen
Abstract
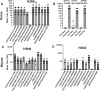
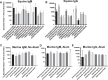
Development of an efficacious universal influenza vaccines remains a long-sought goal. Current vaccines have shortfalls such as mid/low efficacy and needing yearly strain revisions to account for viral drift/shift. Horses undergo bi-annual vaccines for the H3N8 equine influenza virus, and surveillance of sera from vaccinees demonstrated very broad reactivity and neutralization to many influenza strains. Subsequently, vaccinating mice using the equine A/Kentucky/1/1991 strain or recombinant hemagglutinin (HA) induced similar broadly reactive and neutralizing antibodies to seasonal and high pathogenicity avian influenza strains. Challenge of vaccinated mice protected from lethal virus challenges across H1N1 and H3N2 strains. This protection correlated with neutralizing antibodies to the HA head, esterase, and stem regions. Vaccinated ferrets were also protected after challenge with H1N1 influenza A/07/2009 virus using whole viral or HA. These data suggest that equine H3N8 induces broad protection against multiple influenzas using a unique antigen that diverges from other universal vaccine approaches.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The vaccine used in this study is based on a veterinary vaccine (FluAvert) manufactured by Merck Sharp & Dohme LLC. However, Merck was not involved in the planning or execution of this study. D.V. and B.S. have a patent granted for the use of A/equine/Kentucky/91 for use as a universal influenza vaccine and are developing it for use for our company Syntherna. J.E.C. has served as a consultant for Luna Labs USA, Merck Sharp & Dohme Corporation, Emergent Biosolutions, a former member of the Scientific Advisory Boards of Gigagen (Grifols), of Meissa Vaccines, and BTG International, is founder of IDBiologics and receives royalties from UpToDate. The laboratory of J.E.C. received unrelated sponsored research agreements from AstraZeneca, Takeda Vaccines, and IDBiologics during the conduct of the study.
Figures

References
LinkOut - more resources
Full Text Sources

