Berberine alleviates chronic pain-induced anxiety-like behaviors by inhibiting the activation of VLT-projecting cACC (Cg2) neurons
- PMID: 39702401
- PMCID: PMC11659571
- DOI: 10.1038/s42003-024-07372-2
Berberine alleviates chronic pain-induced anxiety-like behaviors by inhibiting the activation of VLT-projecting cACC (Cg2) neurons
Abstract
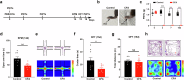
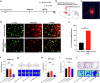
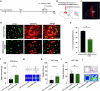
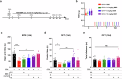
Chronic pain is often accompanied by anxiety, and gradually increasing anxiety makes the pain itself more protracted. Berberine has been found to be able to cross the blood-brain barrier to treat psychiatric disorders, but its neurocirculatory mechanisms remain unclear. Here, we found that neurons in cingulate area 2 (Cg2) of the caudal anterior cingulate cortex (cACC), but not in Cg1 of the cACC, projected to the ventral lateral thalamus (VLT). Next, we induced chronic inflammatory pain by plantar injection of complete Freund's adjuvant (CFA) and observed stable anxiety-like behaviors until two weeks postinjection. We specifically activated VLT-projecting cACC (Cg2) neurons in one-week-old CFA-induced mice without anxiety-like behaviors and in normal control mice to induce anxiety-like behaviors. We inhibited the activation of VLT-projecting cACC (Cg2) neurons in two-week-old CFA-treated mice with anxiety-like behaviors and observed that their anxiety-like behaviors were alleviated. On this basis, we further screened the effective dose of berberine for anxiolysis in two-week-old CFA-treated mice. We observed that the effective dose of berberine obtained above decreased the activity of VLT-projecting cACC (Cg2) neurons. The activation of VLT-projecting cACC (Cg2) neurons abrogated the anxiolytic effect of berberine in two-week-old CFA-treated mice.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures
Similar articles
-
Glutamate transporter activator LDN-212320 prevents chronic pain-induced cognitive impairment and anxiety-like behaviors in a mouse model.Behav Brain Res. 2025 Mar 28;482:115440. doi: 10.1016/j.bbr.2025.115440. Epub 2025 Jan 22. Behav Brain Res. 2025. PMID: 39848593
-
Solanesol Ameliorates Anxiety-like Behaviors via the Downregulation of Cingulate T Cell-Restricted Intracellular Antigen-1 in a Complete Freund's Adjuvant-Induced Mouse Model.Int J Mol Sci. 2024 Sep 21;25(18):10165. doi: 10.3390/ijms251810165. Int J Mol Sci. 2024. PMID: 39337650 Free PMC article.
-
A neural circuit associated with anxiety-like behaviors induced by chronic inflammatory pain and the anxiolytic effects of electroacupuncture.CNS Neurosci Ther. 2024 Apr;30(4):e14520. doi: 10.1111/cns.14520. Epub 2023 Nov 29. CNS Neurosci Ther. 2024. PMID: 38018559 Free PMC article.
-
CCL2 Potentiates Inflammation Pain and Related Anxiety-Like Behavior Through NMDA Signaling in Anterior Cingulate Cortex.Mol Neurobiol. 2024 Aug;61(8):4976-4991. doi: 10.1007/s12035-023-03881-z. Epub 2023 Dec 29. Mol Neurobiol. 2024. PMID: 38157119
-
A systematic review of the pain-related emotional and cognitive impairments in chronic inflammatory pain induced by CFA injection and its mechanism.IBRO Neurosci Rep. 2025 Feb 27;18:414-431. doi: 10.1016/j.ibneur.2025.02.015. eCollection 2025 Jun. IBRO Neurosci Rep. 2025. PMID: 40124113 Free PMC article. Review.
Cited by
-
Exploring the Potential of Dietary Supplements to Alleviate Pain Due to Long COVID.Nutrients. 2025 Apr 7;17(7):1287. doi: 10.3390/nu17071287. Nutrients. 2025. PMID: 40219044 Free PMC article. Review.
References
-
- Cohen, S. P., Vase, L. & Hooten, W. M. Chronic pain: an update on burden, best practices, and new advances. Lancet397, 2082–2097 (2021). - PubMed
-
- Gureje, O. Comorbidity of pain and anxiety disorders. Curr. Psychiatry Rep.10, 318–322 (2008). - PubMed
-
- Carleton, R. N. et al. Anxiety-related psychopathology and chronic pain comorbidity among public safety personnel. J. Anxiety Disord.55, 48–55 (2018). - PubMed
-
- Coakeley, S., Martens, K. E. & Almeida, Q. J. Management of anxiety and motor symptoms in Parkinson’s disease. Expert Rev. Neurother.14, 937–946 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

