Rationale and design of a large trial of perioperative ketamine for prevention of chronic post-surgical pain
- PMID: 39702421
- PMCID: PMC11660817
- DOI: 10.1186/s13063-024-08672-y
Rationale and design of a large trial of perioperative ketamine for prevention of chronic post-surgical pain
Abstract
Background: Chronic post-surgical pain (CPSP) is recognised as one of the most common and debilitating complications of major surgery. Progression from acute to chronic pain after surgery involves sensitisation of central nervous system pathways with the N-methyl-D-aspartate (NMDA) receptor having a central role. Ketamine is a potent, non-selective NMDA antagonist commonly used for management of acute postoperative pain. Inconsistent but largely supportive evidence from small trials of a preventative effect of perioperative ketamine on CPSP risk suggests that a confirmative large trial is needed.
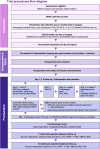
Methods: The ROCKet (Reduction Of Chronic Post-surgical Pain with Ketamine) Trial is a multicentre, double-blind, placebo-controlled, individually randomised superiority trial conducted in 36 hospitals across Australia, New Zealand, and Hong Kong. The trial aims to recruit 4884 patients undergoing abdominal, thoracic, or major orthopaedic surgery. Eligible participants are randomised equally to perioperative intravenous ketamine or placebo for up to 72 h. Incidence of pain in the area of the index surgery is measured by structured telephone interview at 3 months (primary trial endpoint) and 12 months. Pain severity, nature, and associated psychological and quality of life indices are measured using the modified Brief Pain Inventory short form, Neuropathic Pain Questionnaire, Kessler K-10 Psychological Distress Scale, Pain Catastrophising Scale, EQ-5D-3L, and measures of healthcare utilisation and costs. The trial is being conducted by the Department of Critical Care, University of Melbourne, and the Australian and New Zealand College of Anaesthetists Clinical Trials Network. The trial is funded by the Australian National Health and Medical Research Council.
Discussion: The ROCKet trial will clarify the effectiveness of ketamine in primary prevention of CPSP. In addition, it will provide high-quality, prospective data on the epidemiology of CPSP which will better inform further research into prevention and management of CPSP.
Trial registration: Australian New Zealand Clinical Trials Registry (ACTRN12617001619336) on the date of 12/11/2017.
© 2024. The Author(s).
Conflict of interest statement
Declarations. Ethics approval and consent to participate: Approval was granted by the Austin Health Human Research Ethics Committee in 2017 (HREC17Austin161). Written, informed consent to participate will be obtained from all participants. Consent for publication: Not applicable. Competing interests: The authors declare that they have no competing interests.
References
-
- Macrae WA. Chronic pain after surgery. Br J Anaesth. 2001;87:88–98. - PubMed
-
- Wicksell R, Olsson G. Predicting and preventing chronic postsurgical pain and disability. Anesthesiology. 2010;113:1260–1. - PubMed
-
- Katz J, Seltzer Z. Transition from acute to chronic postsurgical pain: risk factors and protective factors. Expert Rev Neurother. 2009;9(5):723–44. - PubMed
-
- Hinrichs-Rocker A, Schulz K, Järvinen I, Lefering R, Simanski C, Neugebauer EA. Psychosocial predictors and correlates for chronic post-surgical pain (CPSP) – a systematic review. Eur J Pain. 2009;13:719–30. - PubMed
-
- Johansen A, Romundstad L, Nielsen CS, Schirmer H, Stubhaug A. Chronic postsurgical pain in a general population: prevalence and predictors in the Tromso study. Pain. 2012;153(7):1390–6. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Research Materials