A miR-219-5p-bmal1b negative feedback loop contributes to circadian regulation in zebrafish
- PMID: 39702498
- PMCID: PMC11659407
- DOI: 10.1038/s42003-024-07309-9
A miR-219-5p-bmal1b negative feedback loop contributes to circadian regulation in zebrafish
Abstract
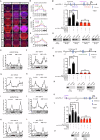
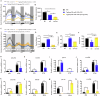
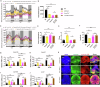
MicroRNAs post-transcriptionally regulate gene expression and contribute to numerous life processes, including circadian rhythms. However, whether miRNAs contribute to zebrafish circadian regulation has not yet been investigated. Here, we showed that mature miR-219-5p, and its three pre-miRNAs, mir-219-1, mir-219-2, and mir-219-3, are rhythmically expressed primarily in Tectum opticum (TeO), Corpus cerebelli (CCe), and Crista cerellaris (CC) of the zebrafish brain. While mir-219-1 and mir-219-2 are regulated by the circadian clock through the E-like box, mir-219-3 is regulated by light via the D-box. Deleting mir-219-1, mir-219-2, or mir-219-3 individually or knocking down miR-219-5p all results in a shortened period of locomotor rhythms and up-regulation of bmal1b. RIP assays with Ago2 and miRNA pull-down assays show that miR-219-5p binds to bmal1b in the RISC. Cell transfection and in Vivo assays show that miR219-5p inhibits bmal1b through binding to its 3'UTR. Further, transcriptome analysis of miR-219-5p knockdown zebrafish adult brain reveals possible roles of miR-219-5p in phototransduction and neuroactive ligand-receptor interaction. Together, our findings demonstrate that mir-219-1, mir-219-2, and mir-219-3 are controlled directly by the circadian clock; and in turn, miR-219-5p contributes to circadian regulation by targeting bmal1b, highlighting a miR-219-5p-bmal1b negative feedback loop in the zebrafish circadian circuit.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

