Chemerin attenuates acute kidney injury by inhibiting ferroptosis via the AMPK/NRF2/SLC7A11 axis
- PMID: 39702678
- PMCID: PMC11659585
- DOI: 10.1038/s42003-024-07377-x
Chemerin attenuates acute kidney injury by inhibiting ferroptosis via the AMPK/NRF2/SLC7A11 axis
Abstract
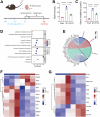
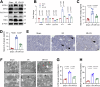
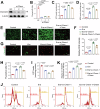
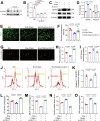
Acute kidney injury (AKI) is a common and life-threatening condition associated with cell death, where ferroptosis plays a critical role. Chemerin, primarily produced in white adipose tissue, has multiple biological functions in renal pathophysiology. However, to date, whether and how chemerin regulates the progression of AKI remain unclear. Here, we found that chemerin expression was reduced in both AKI model mice and cells. Similarly, serum chemerin levels were also decreased in AKI patients. The administration of recombinant chemerin improves renal function in ischemia-reperfusion (I/R) model mice. Chemerin significantly attenuates ferroptosis in kidneys. In TCMK-1 cells, chemerin knockdown further aggravates ferroptosis. Mechanistically, chemerin activates AMP-activated protein kinase (AMPK), which induces the phosphorylation of nuclear factor erythroid 2-related factor 2 (NRF2) in renal tubular cells. Subsequently, NRF2 translocates into the nucleus, where it stimulates the expression of cystine/glutamate antiporter solute carrier (SLC7A11). As a result, cystine uptake and glutathione (GSH) biosynthesis in renal tubular cells were increased, which confers cells with higher capacity against ferroptosis. Overall, our findings indicate that chemerin plays a protective role in AKI by repressing ferroptosis in renal tubular cells, which is likely due to the activation in the AMPK/NRF2/SLC7A11 axis.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: The authors declare no competing interests.
Figures




References
-
- See, E. J. et al. Long-term risk of adverse outcomes after acute kidney injury: a systematic review and meta-analysis of cohort studies using consensus definitions of exposure. Kidney Int.95, 160–172 (2019). - PubMed
-
- Bucaloiu, I. D. et al. Increased risk of death and de novo chronic kidney disease following reversible acute kidney injury. Kidney Int.81, 477–485 (2012). - PubMed
-
- Schetz, M., Gunst, J., De Vlieger, G. & Van den Berghe, G. Recovery from AKI in the critically ill: potential confounders in the evaluation. Intensive Care Med.41, 1648–1657 (2015). - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

