NIS-Seq enables cell-type-agnostic optical perturbation screening
- PMID: 39702735
- PMCID: PMC12339361
- DOI: 10.1038/s41587-024-02516-5
NIS-Seq enables cell-type-agnostic optical perturbation screening
Abstract
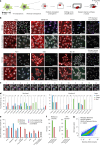
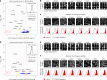
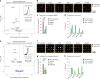
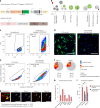
Optical pooled screening offers a broader-scale alternative to enrichment-based perturbation screening, using fluorescence microscopy to correlate phenotypes and perturbations across single cells. Previous methods work well in large, transcriptionally active cell lines, because they rely on cytosolic detection of endogenously expressed barcoded transcripts; however, they are limited by reliable cell segmentation, cytosol size, transcriptional activity and cell density. Nuclear In-Situ Sequencing (NIS-Seq) expands this technology by creating bright sequencing signals directly from nuclear genomic DNA to screen nucleated cells at high density and high library complexity. By inserting an inverted phage promoter downstream of the single guide RNA (sgRNA), many RNA copies of the sgRNA can be generated and sequenced independently of cellular transcription. In this study, we benchmarked NIS-Seq across eight cell types from two species and performed four genome-scale optical perturbation screens, identifying key players of inflammation-related cellular pathways. Finally, we performed a small-scale pooled optical screen in primary human macrophages from blood of healthy donors and demonstrated barcode identification in lentivirally transduced human skin tissue.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: C.I.F., P.K. and J.L.S.-B. are inventors on a patent application related to nuclear in situ sequencing and pooled optical screening. J.L.S.-B. is a co-founder and shareholder of LAMPseq Diagnostics and ions.bio. E.L. and F.I.S. are co-founders and consultants of Odyssey Therapeutics. E.L. is a co-founder of Beren Therapeutics, Metaimmune Therapeutics and IFM Therapeutics. The other authors declare no competing interests.
Figures

References
-
- Carette, J. E. et al. Haploid genetic screens in human cells identify host factors used by pathogens. Science326, 1231–1235 (2009). - PubMed
MeSH terms
LinkOut - more resources
Full Text Sources
Research Materials

