Thermoresponsive gel embedding extracellular vesicles from adipose stromal cells improves the healing of colonic anastomoses following irradiation in rats
- PMID: 39702754
- PMCID: PMC11659299
- DOI: 10.1038/s42003-024-07364-2
Thermoresponsive gel embedding extracellular vesicles from adipose stromal cells improves the healing of colonic anastomoses following irradiation in rats
Abstract
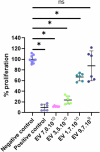
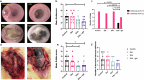
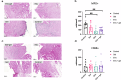
Anastomotic leak occurrence is a severe complication after colorectal surgery. Considering the difficulty of treating these leaks and their impact on patient care, there is a strong need for an efficient prevention strategy. We evaluated a combination of extracellular vesicles (EVs) from rat adipose-derived stromal cells with a thermoresponsive gel, Pluronic® F127 (PF-127) to prevent anastomotic leaks. The pro-regenerative and immunomodulatory potencies of EVs are assessed in vitro. In vivo efficacy are assessed in rat with a colonic anastomosis model after irradiation. Endoscopic, anatomical and histological data show a consistent effect of EVs + gel on the healing of colonic anastomosis. These results are illustrated by a smaller anastomotic ulcer size, less fibrosis and less inflammatory infiltrations in the EVs + gel group. This multi-modal investigation is the first to point-out the translational potential of EVs combined with PF-127 for the healing of high-risk colorectal anastomosis.
© 2024. The Author(s).
Conflict of interest statement
Competing interests: H.A., N.M., G.P., A.C.S., C.D., A.C., A.B., B.M., A.M., O.C., I.A.F. declare no competing interest. G.R. received personal fees from FUJIFILM, MEDTRONIC, and grants from NORGINE. F.G., A.S., C.W., and G.R. are co-founders of the spin-off Evora Biosciences. A.S. and C.W. are co-founders of the spin-off EverZom.
Figures




References
-
- Siegel, R. L., Miller, K. D. & Jemal, A. Cancer statistics, 2018: cancer statistics, 2018. CA Cancer J. Clin.68, 7–30 (2018). - PubMed
-
- Glynne-Jones, R., Wyrwicz, L. & Tiret, E. et al. Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol.28, iv22–iv40 (2017). - PubMed
-
- Penna, M., Hompes, R. & Arnold, S. et al. Incidence and risk factors for anastomotic failure in 1594 patients treated by transanal total mesorectal excision: results from the international TaTME registry. Ann. Surg.269, 700–711 (2019). - PubMed
-
- Alves, A. Postoperative mortality and morbidity in French patients undergoing colorectal surgery: results of a prospective multicenter study. Arch. Surg.140, 278 (2005). - PubMed
-
- Krarup, P. M., Nordholm-Carstensen, A., Jorgensen, L. N. & Harling, H. Anastomotic leak increases distant recurrence and long-term mortality after curative resection for colonic cancer: a nationwide cohort study. Ann. Surg.259, 930–938 (2014). - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

