Changes in Respiratory Syncytial Virus-Associated Hospitalisations Epidemiology After Nirsevimab Introduction in Lyon, France
- PMID: 39702765
- PMCID: PMC11658964
- DOI: 10.1111/irv.70054
Changes in Respiratory Syncytial Virus-Associated Hospitalisations Epidemiology After Nirsevimab Introduction in Lyon, France
Abstract
Background: Respiratory Syncytial Virus (RSV) is a major health concern, particularly for infants. In France, Nirsevimab, a long-acting monoclonal antibody to prevent RSV-associated lower respiratory tract infections (LRTI) was available from September 2023. We described RSV-associated LRTI hospitalisations during the 2023-2024 season among infants younger than six months born at the Hospices Civils de Lyon (HCL), and evaluated the effectiveness of Nirsevimab against RSV-LRTI hospitalisation.
Methods: This observational study included infants born and hospitalised at the HCL during the 2023-2024 season, along with pre-COVID-19 and 2022-2023 seasons. Information on Nirsevimab immunisation status, clinical and perinatal variables were collected through routine care. Infants' characteristics and incidence rate of hospitalisation per 100 births during 2023-2024 were compared with the historical periods overall and by delay between birth and the onset of the RSV season. Nirsevimab effectiveness was computed by the screening method.
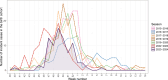
Results: During the 2023-2024 season, 83 infants younger than six months were hospitalised with an RSV-associated LRTI. Compared with the historical periods (640 pre-COVID-19 and 123 in 2022-2023), these infants were older. Incidence rate for infants born during the period when immunisation was available were lower than the previous seasons; incidence rate ratios were 0.45 (95% confidence interval [CI]: 0.33; 0.62) in 2023-2024 compared with pre-COVID-19 period and 0.53 (95%CI: 0.36; 0.77) compared with 2022-2023 season. Nirsevimab effectiveness was 78.3% (95%CI: 55.9; 89.5) with a coverage of 79.3% in the two main HCL maternities.
Conclusions: High Nirsevimab coverage and effectiveness were estimated in a real-world setting. A change in the age distribution of RSV-associated LRTI hospitalisations in 2023-2024 was noted compared with historical seasons.
Keywords: Beyfortus; LRTI; Nirsevimab; RSV; effectiveness; monoclonal antibody; paediatrics; real‐world evidence.
© 2024 The Author(s). Influenza and Other Respiratory Viruses published by John Wiley & Sons Ltd.
Conflict of interest statement
MCN reports grants from Sanofi; and personal fees from Pfizer and Sanofi. EJ reports personal fees from Sanofi. None declared for the other authors.
Figures

References
-
- Mazur N. I., Caballero M. T., and Nunes M. C., “Severe Respiratory Syncytial Virus Infection in Children: Burden, Management, and Emerging Therapies,” Lancet 404, no. 10458 (2024): 1143–1156. - PubMed
-
- Zuurbier R. P., Bogaert D., De Steenhuijsen Piters W. A. A., et al., “Asymptomatic Viral Presence in Early Life Precedes Recurrence of Respiratory Tract Infections,” Pediatric Infectious Disease Journal 42, no. 1 (2023): 59–65. - PubMed
-
- European Medicines Agency . Beyfortus; nirsevimab, https://www.ema.europa.eu/en/medicines/human/EPAR/beyfortus#:~:text=Beyf....
-
- Direction Générale de la Santé . Ouverture des commandes pour le médicament Beyfortus (nirsevimab), https://sante.gouv.fr/IMG/pdf/dgs‐urgent_no2023‐16_‐_ouverture_des_comma....
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

