Evaluating gait and postural responses to subthalamic stimulation and levodopa: A prospective study using wearable technology
- PMID: 39702816
- PMCID: PMC11658965
- DOI: 10.1111/ene.16580
Evaluating gait and postural responses to subthalamic stimulation and levodopa: A prospective study using wearable technology
Abstract
Background: The efficacy of subthalamic stimulation on axial signs of Parkinson's disease (PD) is debated in the literature. This study delves into the dynamic interplay of gait and posture, specifically probing their nuanced response to subthalamic stimulation and levodopa.
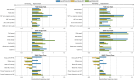
Methods: We used wearable sensor technology to examine alterations in the spatiotemporal parameters of gait and posture in individuals with PD before and 6 months after subthalamic deep brain stimulation (STN-DBS) surgery. Thirty-three subjects with PD were evaluated in two pre-operative and four post-operative conditions comprising OFF/ON medication and stimulation states. Standardized response mean (SRM) values were calculated to assess treatment responsiveness.
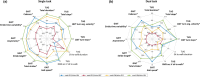
Results: Significant improvements in spatiotemporal gait parameters, including speed, stride length, cadence, and turning, were observed following STN-DBS surgery. Quantitatively, stimulation outperformed levodopa in enhancing gait speed, stride length, and turning, as indicated by SRM. Levodopa moderately improved stride time variability and asymmetry, while stimulation alone demonstrated limited efficacy. Postural parameters exhibited minimal change following STN-DBS, although stimulation showed a slight benefit in certain postural aspects.
Conclusion: Our findings suggest positive effects of stimulation and levodopa on gait and postural parameters, with STN-DBS demonstrating superior efficacy in enhancing gait speed, stride length, and turning. However, gait variability remains unaddressed by current therapies, highlighting the need for novel treatments targeting regions beyond the basal ganglia.
Keywords: Parkinson's disease; deep brain stimulation; inertial measurement units; kinematic analysis; neuromodulation.
© 2024 The Author(s). European Journal of Neurology published by John Wiley & Sons Ltd on behalf of European Academy of Neurology.
Conflict of interest statement
Figures


References
-
- Vitek JL, Jain R, Chen L, et al. Subthalamic nucleus deep brain stimulation with a multiple independent constant current‐controlled device in Parkinson's disease (INTREPID): a multicentre, double‐blind, randomised, sham‐controlled study. The Lancet Neurology. 2020;19:491‐501. doi: 10.1016/S1474-4422(20)30108-3 - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

